J Sci Discov (2017); 1(2):jsd17018; DOI:10.24262/jsd.1.2.17018; Received August 30th, 2017 Revised September 25th, 2017 Accepted October 02nd, 2017 Published October 05th,
The Significance of Pancreatic Acinar Metaplasia at the Lower Esophagus
Ammar Kamil1,*, Nisar Ahmed2
1Clinical Researcher, University of Nebraska Medical Center, Omaha, NE 68198.
2 Chief of Gastroenterology, Park Plaza Hospital, Houston, TX 77004.
* Correspondence: Ammar Kamil M.D., University of Nebraska Medical Center, Omaha, NE 68198. E-mail: ammar.kamil@unmc.edu.
Abstract
The significance of Pancreatic Acinar Metaplasia (PAM) at the lower esophagus has been investigated in a 41-years-old female suffered from gastroesophageal reflux for several years. Erosive esophagitis was found on biopsies by esopahgogastroduodenoscopy. The presence of PAM was shown after two years without any evidence of Barrett Esophagus (BE). However, BE was shown after four years which highlights the possibility of PAM to exacerbate the development of BE. This case indicates that the development of PAM is probably related to the long-standing inflammation in addition to the presence of a genetic factor. Moreover, the rapid development of BE is possibly due to the pancreatic enzymes (like amylase, lipase, and trypsinogen) secreted from the pancreatic acini.
Keywords: Pancreatic acinar metaplasia, Barrett Esophagus, Gastro-esophagus reflux, Pdx-1.
Background
The significance of Pancreatic Acinar Metaplasia (PAM) is still not established well. The prevailing opinion in many clinical studies which is still adopted by many pathologists suggested that PAM is an incidental finding,[1,2] which we doubt, depending on several published studies [3,4,5,6] and according to our observations in the case that we are presenting here, which provide a preponderance of evidence supports the significance of PAM at the lower esophagus.
Case Presentation
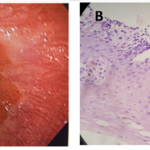
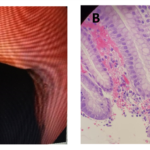
A 41-years-old Hispanic woman presented with a history of acid reflux symptoms in form of repeated attacks of heartburn not responding to antacids which woke her up several times at night, for several years. She tried several over the counter medications including H2-receptor antagonist but it did not help. An esopahgogastroduodenoscopy (EGD) biopsy from distal esophagus showed a (Grade A) erosive esophagitis according to Los Angles classification (Fig. 1-A and 1-B). Pantoprazole 40 mg daily a.m. was prescribed. She did well on it but the patient stopped taking it when she felt better. The patient came back after 2 years, with a recurrence of acid reflux symptoms. This time the EGD biopsy showed a new development of PAM in addition to erosive esophagitis (Fig. 2-A and 2-B). She responded to the pantoprazole when it was recommended. Again the patient did not show up for follow-up for nearly 4 years. After that long period, she came back with severe gastroesophageal reflux disease symptoms. The symptoms were resistant to several medicines that she tried on her own. A repeat EGD and biopsy showed that the patient had developed BE (Fig. 3-A and 3-B).
Figure 1. A lower esophagus. Endoscopic view (A) shows evidence of gastroesophageal reflux disease. Microscopic view (B) shows histological changes compatible with reflux esophagitis. (Hematoxylin and eosin; original magnification × 200).
Figure 2. A lower esophagus. Endoscopic view (A) shows what looked like gastroesophageal reflux disease. Microscopic view (B) shows pancreatic acinar metaplasia. (Hematoxylin and eosin; original magnification × 200).
Figure 3. A lower esophagus. Endoscopic view (A) shows possible early Barrett's Esophagus. Microscopic view (B) confirmed the diagnosis of Barrett’s Esophagus. (Hematoxylin and eosin; original magnification × 200).
Materials and Methods
A total of four biopsies were obtained during every endoscopy from the distal esophagus (3 cm above the gastroesophageal junction). Each esophageal biopsy fragment was collected, labeled separately and sent to the pathology lab for examination.
Discussion
Metaplasia is defined as replacement of one adult type epithelium by another, similarly mature epithelium.[7] The term of metaplasia signifies that the transformation process takes place in postnatal life, that it consists of a tissue that appears in a place it is not normally found and that it can be part of both physiological and pathological processes. Some researchers consider it a process of adapting structures to new conditions in the local environment.[8]
Several theories have been proposed to explain the pathogenesis and occurrence of pancreatic metaplasia. The most tenable theory implicates that during the development of normal pancreas from several evaginations, originating from the wall of the primitive duodenum, one or more evaginations may remain in the bowel wall. Migration of this embryonic remnant along with the development of the gastrointestinal tract gives rise to the ectopic pancreatic tissue.[9] Another theory suggests that during embryogenesis pancreatic metaplasia of the endodermal tissues localized in the gastric submucosa may occur.[9] Histologically, Heinrich in 1909 classified the pancreatic metaplastic tissue which was modified later by Gaspar Fuentes et al into 4 types: type 1, composed by acini, duct and islets similar to those seen in normal pancreas; type 2, composed of ducts only; type 3, consists of acini only (exocrine pancreas); type 4, composed of islets only (endocrine pancreas) [10]. The usual location is in the stomach in 25%-38% of the cases, duodenum in 17%-36%, jejunum in 15%-21.7% and rare in the esophagus.[11]
Pancreatic acinar metaplasia (PAM) is readily identified histologically as cells with basophilic cytoplasm in the basal part, centrally placed nuclei and acidophilic granular cytoplasm in the luminal parts. The cells can be arranged in a single acinus or multiple acini forming small lobuli and they express pancreatic secretory proteins.[12]
Some suggestions indicated an association of PAM with chronic inflammation and BE , so it will be either acquired or increased with gastro-esophageal reflux [3,4,5,6] and others go with the congenital and/or incidental finding. [1,2] But still these studies do not exclude the possibility that PAM might be an effect of certain exposures, even if it would be congenital [4]. What supported the latter possibility is the finding of pancreatic metaplasia inform of foci of pancreatic acini in the human liver; in association with severe post-hepatic cirrhosis, which was not noticed previously.[13]
All authors agree that the islands of acinar epithelial cells produce pancreatic enzymes like amylase, lipase and trypsinogen. Metaplastic cells, through the secretions they produce, can alter the local pH level and can favor the apparition of inflammation.[7,8,14,15]
On the other hand, from a genetic perspective, it has been observed that Pancreatic–duodenal homeobox gene 1 (Pdx-1); which is a homeodomain-containing transcription factor, is essential for pancreatic development and pdx-1 knockout mice found to develop pancreatic agenesis.[16] Furthermore, overexpression of Pdx-1 in cells from neighboring regions of the endoderm was found to induce a pancreatic phenotype and to enhance the ability of these tissues to convert into pancreatic cells.[13]
Conclusions
The inflammatory process from the long-standing erosive esophagitis may be the cause of developing PAM at the lower esophagus.
The pancreatic enzymes secreted from the pancreatic acini may be the underlying cause of rapid development of BE.
Learning Points
• PAM at the lower esophagus is an important pathological finding that should be aware of and a close follow-up is highly recommended.
• PAM at the lower esophagus is an important pathological finding that should be aware of and a close follow-up is highly recommended.
• The presence of a genetic factor in addition to a pathological factor may be the direct cause of developing PAM at the lower esophagus.
• PAM may lead to rapid development of BE, through the secretions that produced by the metaplastic cells, which can alter the local pH level and can favor inflammation.
• This case report opens the door for further studies on the significance of PAM.
Conflict of interest
Authors declare none.
Acknowledgments
We would like to thank the pathology department at the Park Plaza Hospital for their help and support.
References
- Popiolek D, Kahn E, Markowitz J, Daum F. Prevalence and pathogenesis of pancreatic acinar tissue at the gastroesophageal junction in children and young adults. arch. Path Lab Med. 2000;124(8)1165-1167.
- Wang HH, Zeroogian JM, Spechler SJ, Goyal RK, Antonioli DA. Prevalence and significance of pancreatic acinar metaplasia at the gastroesophageal junction. Am J Surg Path. 1996;20:1507–1510.
- Håkansson HO, Mellblom L, Johansson J, Bjartell A, Borgström A. Synthesis and localization of pancreatic secretory proteins in pancreatic acinar-like metaplasia in the distal part of the oesophagus. pancreatic acinar metaplasia: another source of pancreatic enzymes. Scand J Gastroenterol. 2003;38(1):10-13.
- Johan J. Barrett’s oesophagus and metaplasia at the oesophgogastric junction. an epidemiological approach. Ph.D Thesis, Department of Medical Epidemiology and Biostatistics. Karoliniska Institute, Stockholm 2006.
- Mendoza-Ramón H, Goldberg J, Slomianski A, Leal G, Ortiz-Hidalgo C. Presence of acinar pancreas in reflux esophagitis and barrett’s esophagus. Rev Gastroenterol Mex. 1998;63(3):143-147.
- Tiszlavicz L, Németh I , Rosztóczy A , Wittmann T. Histopathology of pancreatic acinar cell metaplasia in barrett’s esophagus. Zeits Gastroenterol. 2006; 44 – A146.
- Krishnamurthy S, Dayal Y. Pancreatic metaplasia in barrett’s esophagus. an immunohisochemical study. Am J Surg Path. 1995; 19(10): 1172-1180 .
- Mogoanta L, Streba CT, Pirici D, Dîrnu R, Oprea B. Pancreatic metaplasia of gastric mucosa associated with gastroduodenal ulcer. Rom J Morph Embryo. 2010; 51(2):365–369.
- Chandan VS, Wang W. Pancreatic heterotopia in the gastric antrum. Arch Path Lab Med.2004;128(1):111-112.
- Trifan A, Târcoveanu E, Danciu M, Huţanaşu C, Cojocariu C, Stanciu C. Gastric heterotopic pancreas: an unusual case and review of the literature. J Gastrointestin Liver Dis. 2012;21(2): 209-212.
- Christodoulidis G, Zacharoulis D, Barbanis S, Katsogridakis E, Hatzitheofilou K. Heterotopic Pancreas in the stomach. a case report and literature review. AM J Gastroenterol. 2007;13(45)6098–6100.
- Lornic E. The columnar lined esophagus: aspects on the assessment of dysplasia and on the relationship with the esophageal submucosal glands. Ph.D Thesis. Lund University, Lund 2015.
- Thowfeequ S, Myatt E, Tosh D. Transdifferentiation in developmental biology, disease, and in therapy. Dev Dyn. 2007;236:3208–3217.
- Takubo K, Arai T, Sawabe M , Miyashita M, Sasajima K, Iwakiri K, Mafune K. Structures of the normal esophagus and barrett’s esophagus. Esoph. 2003;1:37–47.
- Westerterp M, Busch O. R. C., Bergman J. J. G. H. M., Ten Kate F. J. W.,Van Lanschot J. J. B. A ‘crackleware’ oesophagus. J Clin Path. 2005; 58: 1325–1327.
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1is required for pancreatic outgrowth and differentiation of the rostral duodenum. Dev. 1996;122(3): 983-995.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/