J Sci Discov (2017); 1(2):jsd17013; DOI:10.24262/jsd.1.2.17013; Received July 16th, 2017, Revised July 27th, 2017, Accepted August 8th, 2017, Published August 15th, 2017.
Partial Gastric Outlet Obstruction associated with “Duodenal Lymphangiectasia”
Ali Hameed Ali1,#, Mustafa Abdulsada1,#, Ahmed Al-Gurairi1,#, Dr. Nisar Ahmed1,*
1. Department of Gastroenterology, Park Plaza Hospital, Houston, TX 77004 USA.
#Ali Hameed Ali, Mustafa Abdulsada1 and Ahmed Al-Gurairi contributed equally to this work.
* Correspondence: Dr. Nisar Ahmed, M.D. F.A.C.G. , Chief of Gastroenterology, Park Plaza Hospital, Houston, TX 77004, USA. Email: nisarahmedmd@yahoo.com.
Abstract
Intestinal lymphangiectasia is a rare disorder characterized by dilated intestinal lacteals, it can present with symptoms of hypoalbuminemia or it can be found accidentally during upper GI endoscopy done for variable reasons.
In this case report, we discuss the possible association between Intestinal lymphangiectasia and symptoms of partial gastric outlet obstruction of undetermined cause.
Introduction
Intestinal lymphangiectasia (IL) is a rare disease and was first reported by Waldmann in 1961 characterized as a disorder with dilated intestinal lacteals that could be found in the duodenum, Jejunum or Ileum. It can be classified into Primary (idiopathic; PIL); and Secondary [1].
Clinically overt IL is of unknown prevalence. Familial forms of Waldmann’s disease have been reported; IL primarily affects children (especially under the age of 3 years) and young adults though may be diagnosed later in adults [2, 3].
IL in adults detected in recent years, however, appears to have few or no clinical features [4]. However, IL might present with symptoms of malabsorption, protein losing enteropathy, lymphopenia and mechanical ileus [1, 7, and 8]. Diagnosis remains dependent on endoscopic changes confirmed by small bowel biopsy showing histological evidence of IL [4].
In this case report, we highlight the possible association between partial gastric outlet obstruction of unclear etiology and duodenal lymphangiectasia.
Case History
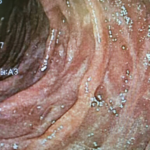
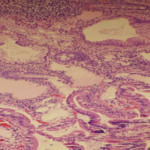
A 39 years old African American patient was referred to us for evaluation of recurrent nausea, vomiting, early satiety of one year duration. On upper endoscopy she was found to have white tipped villi (Fig. 1) in the duodenum which on biopsy showed evidence of lymphangiectasia (Fig. 2). Mild chronic gastritis was also found. No Helicobacter pylori was detected. CT scan of chest and abdomen were negative. She did have history of laparoscopic cholecystectomy three years ago. Patient did well on Pantaprazole 40 mg. daily AM, five small meals of mechanical soft diet, and metoclopramide 5mg. three times a day.
Discussion
IL is a relatively rare disorder that usually manifests itself through intestinal malabsorption. Its worldwide incidence and prevalence are unknown. The disease affects males and females equally, and there is no racial predilection [11, 12]. The disease can be primary (congenital), where there is malformation within the lymphatic channels leading to their blockage, and the condition is usually diagnosed within the first decade of life, although some cases can be asymptomatic and discovered accidentally by upper endoscope for various reasons later in life. The condition can also be secondary to other disease states, such as constrictive pericarditis, lymphoma, sarcoidosis and scleroderma, and it can affect older adults. The first manifestations are usually persistent diarrhea and peripheral edema that happen due to malabsorption, lymphocytopenia, hypogammaglobulinemia and hypoproteinemia [9,13]. The mechanisms for enteric protein loss in IL are not well understood, although an increase in the pressure of the lymph channels has been suggested to be a possible cause of protein loss [5, 10, and 13].
The prevalence of incidentally found duodenal lymphangiectasia, in a retrospective study, confirmed by histology was 1.9% among cases undergoing routine diagnostic upper gastrointestinal endoscopies for various reasons. The prevalence of duodenal lymphangiectasia could be 3.2%, if all individuals in whom duodenal lymphangiectasia was suspected endoscopically were included [5].
The prevalence of histologically proven duodenal lymphangiectasia from the prospective study was 9.0 % among asymptomatic individuals undergoing upper gastrointestinal endoscopies for routine health check-ups. In conclusion, duodenal lymphangiectasia without clinical evidence of malabsorption is not uncommon among cases under-going routine upper gastrointestinal endoscopy and repeat endoscopy or biopsy is not warranted in the absence of clinical evidence of malabsorption [5].
In the above mentioned study, among 52 individuals in whom duodenal lymphangiectasia was endoscopically suspected and from whom endoscopic biopsies were taken, 47 (90.4%) showed characteristic histologic findings of dilated lymphatic vessels in the mucosa and submucosa. Therefore, a careful endoscopic observation is usually sufficient for the diagnosis of lymphangiectasia without pathologic examination with endoscopic biopsy specimen [5].
Gross endoscopic findings and histopathology of intestinal biopsy specimens (Fig. 2) confirm the diagnosis of IL. Macroscopic abnormalities are characterized by creamy yellow plaques of intestinal villi corresponding to marked dilation of the lymphatics within the intestinal mucosa. There is patient to patient variation in densities and size which ranges from millimeters to centimeter. Histological examination confirms the presence of lacteal juice, dilated mucosal, submucosal and, to lesser extent, serosal lymphatic vessels with polyclonal normal plasma cells [1, 6] .Intestinal wall-thickening leads to lumen narrowing that slows the passage of food which could manifest as mechanical obstruction in some cases [7]. Hypothetically, we suggest that these pathological changes might lead to partial gastric outlet obstruction, particularly when it is found in the first part of the duodenum.
Gastric outlet obstruction (GOO, also known as pyloric obstruction) is not a single entity; it is the clinical and pathophysiological consequence of any disease process that produces a mechanical impediment to gastric emptying. Clinical entities that can result in GOO generally are categorized into two well-defined groups of causes: benign like peptic ulcer disease and malignant like pancreatic cancer [14]. Symptoms suggesting gastric outlet obstruction include recurrent episodes of emesis with large volumes of vomit containing undigested food; persistent bloating or fullness after eating; and early satiety. Weight loss, dehydration, and a hypochloremic, hypokalemic metabolic alkalosis may result; a tympanic epigastric mass representing the dilated stomach with visible gastric peristalsis also may be observed [8].
Fig 1: upper endoscopy, white tipped villi
Fig 2: Histopathology, evidence of lymphangiectasia with Mild chronic gastritis, marked dilation of the lymphatics
Conclusion
We conclude that Partial gastric outlet obstruction of unclear etiology might be attributed to duodenal lymphangectasia. So we recommend considering this pathology as one of the causes of partial gastric outlet obstruction. Moreover, we encourage reporting similar case scenarios so we will come to solid base to confirm our consideration.
Conflict of interest
None
Acknowledgments
None
References
1. Fang YH, Zhang BL, Wu JG, Chen CX. A primary intestinal lymphangiectasia patient diagnosed by capsule endoscopy and confirmed at surgery: A case report. World J Gastroenterol 2007; 13(15): 2263-2265.
2. Boursier V, Vignes S. Lymphangiectasies intestinales primitives (maladie de Waldmann) révélées par un lymphœdème des membres. J Mal Vasc. 2004; 29:103–106.
3. Tift WL, Lloyd JK. Intestinal lymphangiectasia long-term results with MCT diet. Arch Dis Child. 1975;50:269–276.
4. Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol 2011; 3(2): 19-23.
5. Kim J H. et al. Clinical significance of asymptomatic duodenal lymphangiectasia …Endoscopy 2009; 41: 510 –515.
6. Chamouard P, Nehme-Schuster H, Simler JM, Finck G, Baumann R, Pasquali JL. Videocapsule endoscopy is useful for the diagnosis of intestinal lymphangiectasia. Dig Liver Dis. 2006; 38:699–703.
7. Lenzhofer R, Lindner M, Moser A, Berger J, Schuschnigg C, Thurner J. Acute jejunal ileus in intestinal lymphangiectasia. Clin Investig. 1993;71:568–571.
8. Lobo B, Casellas F, de Torres I, Chicharro L, Malagelada JR. Usefulness of jejunal biopsy in the study of intestinal malabsorption in the elderly. Rev Esp Enferm Dig. 2004; 96:259–264.
9. Toskes P: Gastrointestinal diseases: malabsorption. Cecil Textbook of Medicine. Edited by: Wyngaarden J, Smith L. 1988, Philadelphia: WB Saunders, 732-745. 18.
10. Mistilis SP, Stephen DD, Skyring AP: Intestinal lymphangiectasia. Mechanism of enteric loss of plasma-protein and fat. Lancet. 1965, 1: 77-81.
11. Lai Y, Yu T, Qiao XY, Zhao LN, Chen QK. Primary intestinal lymphangiectasia diagnosed by double-balloon enteroscopy and treated by medium-chain triglycerides: a case report. J Med Case Rep. 2013; 7:19.
12. Rashmi MV, Niranjana Murthy B, Rani H, Kodandaswamy CR, Arava S. Intestinal lymphangiectasia – a report of two cases. Indian J Surg. 2010; 72:149–151.
13. Jeffries GH, Chapman A, Sleisenger MH: Low-fat diet in intestinal lymphangiectasia. Its effects on albumin metabolism. N Engl J Med. 1965, 270: 761-766.
14. Andersson A, Bergdahl L. Carcinoid tumors of the appendix in children. A report of 25 cases. Acta Chir Scand. 1977. 143(3):173-5.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/