J Sci Discov (2017);1(1):jsd17010;DOI:10.24262/jsd.1.1.17010; Received May 29th,2017,Accepted June 12nd,2017,Published June 22nd,2017.
Bio-adsorption of Heavy Metals from Aqueous Solutions by Natural and Modified Non-living Roots of Wild Scorzonera incisa DC.
Parisa Ziarati1*, Iman Mohsenin Moshiri2,Peyman Sadeghi2
1 Department of Medicinal Chemistry,Faculty of Pharmacy,Pharmaceutical Sciences Branch,Islamic Azad University,Tehran – Iran (IAUPS)
2Nutrition & Food Sciences Research Center,Pharmaceutical Sciences Branch,Islamic Azad University,Tehran-Iran (IAUPS)
* Correspondence:Parisa Ziarati,Islamic Azad University,Pharmaceutical Sciences Branch (IAUPS),Faculty of Pharmacy,Nutrition and Food Sciences Research Center. No 99,Yakhchal,Gholhak,Dr.Shariati,Tehran-Iran.Email:ziarati.p@iaups.ac.ir.Tel:+98-21-22640051;Fax:+98-21-22633986.
Abstract
Bio-adsorption capacity of Alapouk or Sheng Asbi as a native name of Scorzonera incisa DC (S.incisa), in a batch system by considering the effects of various parameters like initial concentration, pH, contact time, temperature, agitation speed and bio-adsorbent dose were studied.The plant with two or more leaves and is wildly grown in Fuladshar–Isfahan in the center of Iran was investigated.S.incisa grows within the short period starting at about middle March and ending late in April and local people drink the infusion of the root as herbal tea for lowering the blood sugar.Different digested contents of infused S.incise root waste was equilibrated with 100 ml of the Cd, Ni, Co, Pb, Zn, Cu solution of known concentration.Parameters such as various pH,temperature,dose (0.1%,0.2%,0.3% and 0.5 mg/L of solution),contact time (1,5,10,15,30,40 min),initial concentration (0.2,0.5,0.7,1.0,3.0 ppm),particle size and agitation speed(100,300,400,800 rpm) were studied.The samples were analyzed by standardized international protocols in Nutrition and Food Sciences Research Center in Pharmaceutical Sciences Branch, Islamic Azad University.The adsorption was solution pH dependent and the maximum adsorption for Co, Cd, and Zn was observed at solution pH of 3.0 while for Ni, Zn and Pb were of 2.5.The amounts of Cd bio-adsorbed increased with increase in dose of both S.incisa root waste and their contact time.A contact time of 30 min was found to be optimum for most of the studied heavy metals.Experimental results show the low cost of root plant waste after infusion was effective for the removal of pollutants from aqueous solution.
Keywords: Scorzonera incise DC root, Removal, Heavy metals, Bio-adsorption, Aqueous Solution.
Introduction
Water pollution is one of the major global problems and it is the leading worldwide cause of deaths and diseases.Pollution of the biosphere with toxic metals has stimulated seriously since the presentation of the Industrial Revolution [1-3].Considerable scientific,economical,and,social values attentions in wetland as a water environment has been made.They are the habitats of various organisms devoted to the gene cache of microbes,plants and animals on the earth.In spite of this fact,momentarily the pollution and degradation of the wetlands are dramatically increasing [3-8].Plentiful activities in conjunction with metal plating,fertilizer industry,metallurgy,mining operations,battery manufacturing and textile dyeing generate enormous volume of wastewater contaminated with various metals[9-12]. Discharging of metallic ions in industrial effluent is of great concern due to their presence and being accumulated as the toxic materials on living species [13].Heavy metals are toxic to aquatic organisms even at very low concentration and the soil and water of many cities especially in the north and south of Iran has been already contaminated [14-17].
In order to reduce heavy metals such as Ni,Co,Pb,Cd,Zn and Cu in the contaminant industrial wastewater samples by higher level than the standard level,an efficient and low cost method needs to be developed.The various methods of removal of heavy metals from industrial wastewater include filtration,chemical precipitation,adsorption,electrodeposition and membrane systems,or even ion exchange process [2].In the discharge of metal ions in industrial effluent using bio-adsorption process has been an area of extensive research because of the presence and accumulation of toxic carcinogenic effect on living species.The most common and harmful heavy metals are aluminium,lead,copper,nickel,chromium and zinc.They are stable elements that cannot be metabolized by the body and get passed up in the food chain to human beings.When waste is disposed into the environment,a further long-term hazard is encountered.There are possibly more problems from these metals,which interfere with normal bodily function,than have been considered in most medical circles.Reviewing allof our vitamins and minerals has shown us that most every substance that is useful can be a toxin or poison,as well.Metals are known primarily and almost exclusively for their potential toxicity in the body,though commercially they may have great advantages [13-16].
Scorzonera species are treated in different folk medicines to combat many diseases,including the illnesses connected with inflammation.Alapouk or Sheng Asbi is the native name of Scorzonera incisa DC,a plant with two or more leaves and is grown in Fuladshar–Isfahan in the center of Iran.The length of its leaves regularly measure a few centimeters.The leaves vary in color from dark green to dark brown.The plant affords beautiful yellow flowers in continuation of its life period.This plant commonly grows at various zones of southern Khorasan,south Khorasan Razavite and Mehrdasht and Lenjan of Isfahan Province.Stem leaves at most c.1/2 size of basal leaves.Capitula 1-2(-4) per stem,(35-) 40-50 mm long.Inner phyllaries c.25 mm [18-34].
It grows within the period starting at about middle March and ending late in April.It is used as an edible vegetable.Its leaves are sometimes cooked before they are served. About 70 years ago the natives of the area ground the nodes of the plant in a mortar and mixed water,wheat or barley flour,some salt and such things to bake bread.Interestingly the other plant (scorzonera paradoxa fisch.&C.A.Mey) which is very similar to S.incise in Khorasan region is called Peyghambar bread,Galvac or Naghoudashak and native people are used to eat it as edible vegetable and mostly cooked it.It has excrescence root in various forms,which is edible as well.Based on a study carried out in scorzonera paradoxa fisch.&C.A.Mey plant,the leaves of this plant contain higher percentages of protein,ash and crude fat compared to those in root[35].Highest rates of energy and crude fiber are found in the root.The leaves contain considerable values of zinc,manganese,chrome,magnesium and cadmium while the root contain significant values of iron,zinc and manganese.Prolonged uses of considerable amounts of this plant bring reduction to blood sugar;however it may result in hepatic injury in diabetic mice.Healthy mice may develop symptoms of mild toxicity with an increase in dosage[36-38].In Iran few studies have been conducted to find effective bio-adsorption of endemic plants for removal or decreasing heavy metals.The objective of current research was investigation of the potential of the Scorzonera incisa DC root,considering as a high-biomass Ni,Cd,Pb,Co,Cu and Zn hyperaccumulator to accomplish as a proper bio-adsorbent these metals from artificial metalliferous media .
Materials and Methods
Sampling Plant
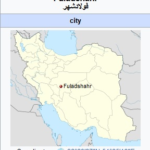
S.incisa plant gathered from Fuladshahr in March and April of 2017 randomly.Fuladshahr is situated 25 kilometers (16 mi) south of Isfahan.Isfahan is located on the main north–south and east–west routes crossing Iran,and was once one of the largest cities in the world[39].No much rain in Fuladshahr all through the year.Mean annual temperature is 15.2 degrees Celsius and man annual precipitation is 154 mm.the soil is clay in plains and it is a combination of sands and soil on mountainous areas.The sampling zone has the following geographical coordinates:32°29′27″N 51°25′16″E,which is shown in figure 1.
Figure 1- Location of S.incisa samples collection
Preparation of S.incisa Roots
The S.incisa roots were dried for a period of one week and then cleaned with distilled water thoroughly and dried at room temperature in the glass dishes.The roots were grounded with the grinding mill.The ground S.incisa roots were sieved and were of particle size 0.25 to 0.4 mm.This was to allow for shorter diffusion path,thus allowing the adsorbate (S.incisa roots) to penetrate deeper into the contaminated water more quickly,resulting in a higher rate of adsorption [40].
The roots of plant were dried at 80º C in an oven for 24 hours.Dry parts were then ground and weighed.Plant samples (approximately 1.0 g) were soaked by citric acid 1% for 1 hour and then digested with concentrated HNO3 and H2O2 [13,41-44].The digested solution was filtered and then analyzed by Flame Emission Spectrophotometer.
Effect of various pH (The pH of studied solution was adjusted with a 0.1M HCl /0.1M NaOH solution);temperature;dose 0.1%,0.2%,0.3% and 0.5 mg/L of solution;contact time 1,5,10,15,30,40 min;initial concentration 0.2,0.5,0.7,1.0,3.0 ppm;particle size mesh>30,mesh<30,mesh>20;and agitation speed 100,300,400,800rpm were studied(Time of each experiment was kept at 40 min.These flasks were shaken on the shaker at different rpm,but the optimum was considered as 400 rpm).
Zinc,Cobalt,Nickel,Cadmium,Lead,Copper and Zinc Determination
For Zinc,Cobalt,Nickel,Lead,Cadmium and Copper and concentrations in powdered root parts of samples were dried in oven for 48 hours at a temperature of 85 °C.The powdered samples then subjected to the acid digestion using nitric acid (65% Merck,Germany),Sulfuric acid (96.5% Merck,Germany) and perchloric acid(70% Sigma- Aldrich).Two gram of air-dried of each homogeneously S.incisa root samples accurately weighed and 20.0 mL of the digestion mixture(3 parts by weight of concentrated nitric acid:2 parts of concentrated Sulfuric acid & 3 parts by weight concentrated perchloric acid) and heated slowly by an oven and then rise the temperature.The remaining dry inorganic residues were dissolved in 25.0 mL of nitric acid and the solution used for the determination of mineral elements.Blanks and samples were also processed and analyzed simultaneously.All the chemicals used were of analytical grade(AR).Standardized international protocols were followed for the preparation of material and analysis of heavy metals contents [45-49].The samples were analyzed by Flame Emission Spectrophotometer,using at six standard solutions for each metal and determination of potassium content was followed by FDA Elemental analysis(ORA LABORATORY MANUAL,2013) [49].Also,periodic testing of standard solutions was performed in order to verify of reliability of the measuring apparatus.The accuracy was checked using quality control test for fungi and their substrate samples to show the degree of agreement between the standard values and measured values;the difference was less than 5%.The samples were analyzed by Flame Emission Spectrophotometer Model AA-6200 (Shimadzu,Japan) using an air-acetylene,flame temperature:2800°C,acetylene pressure:0.9–1.0 bar,air pressure:4.5–5 bar,reading time:1–10 sec (max 60 sec),flow time:3-4 sec (max 10 sec).
Statistical Method
One-parametric Kruskal–Wallis/Mann–Whitney U tests were applied to compare differences between objects.Non-parametric multiple comparison test (Dunn’s test) was performed to determine statistical significance of results at α = 0.05.The GLM procedure was used for analysis of different metal treatments with means separated by Duncan’s multiple range test at p<0.05.The CORR procedure was used for correlation analysis with means separated at p<0.05.
Results & Discussion
The main content of heavy and mineral elements:Zinc,Cobalt,Cadmium,Copper,Lead and Nickel in presence of S.incisa root samples collected from Fuladshahr-Isfahan,Iran are shown in Table 1 and figures 2-4.The samples were analyzed by wet digestion method and standardized international protocols were followed for the preparation of material and analysis of heavy metals contents and analyzed by Atomic Absorption Spectrophotometer in Research Laboratory in Pharmaceutical Sciences Branch,Islamic Azad University.The data obtained from chemical analyses,mean values were calculated and are given in the table 1,with their standard deviations.
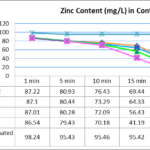
Fig.2 shows the bio-adsorption of Zinc by S.incisa root samples as a function of time.Initial Zinc concentration was 100 mg/L and bio-absorbents dose of 0.1,0.2,0.3 and 0.5 mg/100ml were used.Fig.2 indicates that rapid adsorption in the initial 30 min for all bio-adsorbent.Mostly,the removal of zinc is rapid,but it gradually decreases with time until it reaches equilibrium.The necessary time to reach this equilibrium is about 30 min.Further increase in contact time for 40 minutes did not show significant increase in bio-adsorption (p >0.05).
Figure 2- Effect of contact time on the removal of Zinc (initial Zinc concentration=100 mg/L, adsorbent dose=0.1, 0.2, 0.3 and 0.5 mg/100 ml, temperature=25 ± 1 ºC, agitation speed= 400 rpm), pH = 3.0
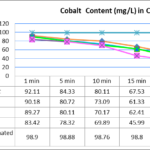
The adsorption of Co at different percentage of bio-adsorbents shows an increase in the adsorption capacity when the 0.3 and 0.5 mg/L concentrations of S.incisa root are increased (Fig.3).Similar trends are observed for all the other concentrations.This indicates that the adsorption reaction is increasing by the surface area in nature.The enhancement in
the adsorption capacity may be due to the chemical interaction between bio-adsorbates and adsorbent,formulation of some new adsorption sites or the expanded percentage of intra-particle dispersion of Co ions into the pores of the adsorbent at higher concentrations.Removal of Cd at pH>4 is zero whereas its removal percentage is very high at pH=3;however,removal percentage of cadmium is significantly low after 10 minutes.Figure 4 shows that,the pH of the solution is a very important parameter for the removal of Cd,in the optimum pH=3 after 30 minutes showed a significant increasing in adsorbing (p < 0.001).
Figure 3- Effect of contact time on the removal of Cobalt (initial Cobalt concentration=100 mg/L, adsorbent dose=0.1, 0.2, 0.3 and 0.5 mg/100 mL, temperature=25 ± 1 ºC, agitation speed= 400 rpm), pH = 3.0
Figure 4- Effect of contact time on the removal of Cd (initial Cadmium concentration=50 mg/L, adsorbent dose=0.1, 0.2, 0.3 and 0.5 mg/100 ml, temperature=25 ± 1 ºC, agitation speed= 400 rpm), pH = 3.0.
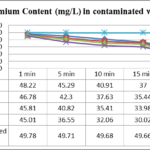
Results in table 1 showed significant difference in Nickel up -taking after 5 minutes by 0.2%,0.3% and 0.5 % bio-adsorbents,but the potential of taking up Nickel by 0.5% was not as much as different from lower percent’s (p> 0.05) .Moreover,time factor of putting adsorbent in contaminated water by different heavy metals (Pb,Ni and Cu) in the study showed significant (p <0.05) and positive correlation with contents of Ni (r = +89 to r = +93),Cu (r = +82 to r = +86 ) and for Pb (r = +90 to r = +85 ) in the contaminated water and S.incisa root respectively.The amounts of Nickel adsorbed increased significantly with increase contact time (p<0.005).
| Pb content(mg/L± SD) | 1 min | 5 min | 10 min | 15 min | 30 min | 40 min |
| 0.1%bio-adsorbent | a97.66±o.11 | a 90.37±0.23 | a 84.81±0.18 | b 71.22±0.16 | c 59.05±0.15 | c 47.21±0.21 |
| 0.2%bio-adsorbent | a 95.45±0.23 | a 84.33±0.17 | b 76.59±0.12 | b 63.29±0.09 | c 49.89±0.10 | c 44.78±0.15 |
| 0.3%bio-adsorbent | a 94.39±0.15 | a 84.39±0.15 | b 76.54±0.16 | b 65.49±0.21 | c 40.32±0.18 | c 40.28±0.16 |
| 0.5%bio-adsorbent | a 93.02±0.14 | b 78.18±0.14 | b 65.32±0.13 | c 49.78±0.13 | d 39.81±0.08 | d 36.48±0.04 |
| Untreated contaminated wastewater | a 99.18±0.05 | a99.01±0.16 | a 98.86±0.17 | a 98.85±0.16 | a 98.69±0.05 | a 98.84±0.07 |
| Cu Content (mg/L ± SD) | 1 min | 5 min | 10 min | 15 min | 30 min | 40 min |
| 0.1%bio-adsorbent | a 94.52±0.15 | b 82.01±0.09 | c 65.44±0.06 | c 59.17±0.14 | d 44.28±0.13 | d 40.17±0.11 |
| 0.2%bio-adsorbent | a 94.44±0.12 | b 80.17±0.09 | c 60.18±0.15 | c 53.07±0.03 | d 40.29±0.13 | d 38.76±0.13 |
| 0.3%bio-adsorbent | a 93.29±0.06 | b 78.43±0.08 | c 59.37±0.09 | c 50.18±0.08 | d 38.22±0.04 | d 36.54±0.12 |
| 0.5%bio-adsorbent | a 91.09±0.14 | b 76.07±0.08 | c 54.23±0.07 | c 48.72±0.06 | d 34.82±0.05 | d 30.11±0.10 |
| untreated contaminated wastewater | a 98.72±0.18 | a 98.54±0.16 | a 98.14±0.05 | a 96.24±0.11 | a 95.89±0.10 | a 94.88±0.12 |
| Ni Content(mg/L ± SD) | 1 min | 5 min | 10 min | 15 min | 30 min | 40 min |
| 0.1% bio-adsorbent | a 95.66±0.14 | a 89.73±0.12 | b 73.44±0.12 | c 60.21±0.10 | c 56.34±0.06 | c 52.39±0.03 |
| 0.2% bio-adsorbent | a 95.03±0.14 | b 80.24±0.11 | c 71.62±0.06 | d 58.43±0.08 | d 50.94±0.06 | d 48.44±0.13 |
| 0.3% bio-adsorbent | a 94.89±0.15 | b 82.01±0.15 | c 72.33±0.12 | d 57.03±0.11 | d 50.11±0.07 | d 49.34±0.11 |
| 0.5% bio-adsorbent | a 95.01±0.09 | b 84.33±0.05 | b 73.02±0.11 | c 56.09±0.15 | c 50.81±0.06 | d 42.23±0.14 |
| untreated contaminated wastewater | a 98.09±0.16 | a 97.56±0.14 | a 98.65±0.13 | a 98.43±0.13 | a 96.78±0.14 | a 96.32±0.16 |
Table 1- Effect of contact time on the removal of Pb, Cu and Ni (initial Lead, Copper and Nickel concentrations =100 mg/L ± SD , adsorbent dose=0.1, 0.2, 0.3 and 0.5 mg/100 mL, temperature=25 ± 1 ºC, agitation speed= 400 rpm), pH = 2.5.
*Mean values with different small letters are significantly different potential of bio-adsorbing for removing heavy metals (p<0.05)
Experimental results showed that the percentage removal Lead and Copper increases with the increasing amount of bio-adsorbent up to 0.3 mg/L
for S.incisa root samples 30 minutes of shaking after .After this dose of adsorbent no significant
change was observed,but for Nickel removing in the dose of 0.2 mg/L after even 5 minutes a significant decreasing of heavy metal is observed,although percentage removal Ni increases with the increasing amount of bio-adsorbent.
Conclusion
A contact time of 30 min was found to be optimum for most of studied heavy metals.Experimental results show low cost of root plant waste after infusion was effective for the removal of pollutants from aqueous solution.The phenomenon of enhancing in percent heavy metals removal with increase in bio-adsorbent doses was related to the availability of more and more adsorbent surfaces for the solutes to adsorb,but after the dose of 0.3 mg/L adsorbent no significant change was observed,which means even by a few amounts of S.incisa root,the removal of heavy metals from contaminated water could be accomplished and this green,cheap and ecofriendly method is recommended.
Acknowledgments
Pharmaceutical Sciences Branch,Islamic Azad University (IAUPS) is gratefully acknowledged.
Conflicts of Interest
None of the authors have any conflicts of interest associated with this study.
References
1.Seifi-Nigje Gheshlagh F,Ziarati P,Arbabi Bidgoli S.Seasonal fluctuation of heavy metal and nitrate pollution in ground water of farmlands in Talesh Gilan,Iran.International Journal of Farming and Allied Sciences.2013;2(20):836-841.
2.Khazaei I,Aliabadi M,Hamed Mosavian HT.Use of Agricultural Waste for Removal of Cr(VI) from Aqueous Solution.Iranian Journal of Chemical Engineering IAChE,2011;8(4):11-23.
3.Ziarati P,MirMohammad-Makki FS, Moslehishad M.Novel Adsorption Method for Contaminated Water by Wild Endemic Almond:Amygdalus scoparia.Bioscience and Biotechnology Research Asia,2016;13(1):147-153.
4.U.S.Department of Health and Human Services.Report on carcinogens.Twelfth edition,Public Health Service,National Toxicology Program;2011.
5.Mance G.Pollution Threat of Heavy Metals in Aquatic Environment,Elsevier Applied Sciences,New York,NY,1987,p372.
6.He J,Chen JP.A comprehensive review on bio-sorption of heavy metals by algal biomass:materials,performances,chemistry,and modeling simulation tools,Bioresour.Technol.2014;160:67–78.
7.Vijayaraghavan K,Yun YS.Bacterial biosorbents and biosorption,Biotechnol. Adv.2008;26 :266–291.
8.Gao J,Zhang Q,Su K,Chen R,Peng Y.Biosorption of acid yellow 17 from aqueous solution by non-living aerobic granular sludge,J.Hazard.Mater,2010;174 (1–3):215–225.
9.loussaief M,Benzina M.Efficiency of natural and acid-activated clays in the removal of Pb(II) from aqueous solutions.J Hazard Mater,2010;178:753–757.
10.Eloussaief M,Kallel N,Yaacoubi A,Benzina M.Mineralogical identification,spectroscopic characterization,and potential environmental use of natural clay materials on chromate removal from aqueous solutions.Chem Eng J,2011;168:1024–1031.
11.Eloussaief M,Hamza W,Kallel N,Benzina M.Wastewaters decontamination:Mechanisms of Pb(II),Zn(II),and Cd(II) competitive adsorption on tunisian smectite in single and multi-solute systems.Environ Prog Sustainable Energy.2012.doi:10.1002/ep.11609.
12.Sdiri A,Higashi T,Chaabouni R,Jamousssi F.Competitive removal of heavy metals from aqueous solutions by montmorillonitic and calcareous clays.Water Air Soil Pollut,2012;223:1191–1204.
13.Ziarati P,Kermanshah A,Moslehishad M.Adsorption Heavy Metal from Contaminated Water by Modified Shell of Wild Endemic Almonds:Amygdalus lycioides and Amygdalus wendelboi.Bioscience and Biotechnology Research Asia,2015;12(3):2451-2457.
14.Albaji A,Ziarati P,Shiralipour R.Mercury and Lead Contamination Study of Drinking Water in Ahvaz,Iran.International Journal of Farming and Allied Sciences.2013;2(19):751-755.
15.Ziarati P,Alaedini S.The Phytoremediation Technique for Cleaning up Contaminated Soil By Amaranthus sp.J Environ Anal Toxicol,2014;4:208.doi:10.4172/2161-0525.1000208.
16.Ziarati P,Zendehdel T,Bidgoli SA.Nitrate Content in Drinking Water in Gilan and Mazandaran Provinces,Iran.J Environ Anal Toxicol,2014;4:219.doi:10.4172/2161-0525.1000219.
17.Shokri F,Ziarati P,Mousavi Z.Removal of Selected Heavy Metals from Pharmaceutical Effluent by Aloe vera L.Biomedical& Pharmacology Journal,2016;9(2):705-713 .
18.Chamberlain D F.Scorzonera L.in Davis,P.H.,Flora of Turkey and the East Aegean Islands,1975;5:102.
19.Heller B D, Heyn CC. Scorzonera bulbipes Boiss.&Hausskn.Conspectus Florae Orientalis,1993;8.
20.Heller CD,Heyn C C.Conspectus Florae Orientalis,1993;8.
21.Rechinger DK H.Scorzonera bulbipes Boiss.& Hausskn.Koelpinia.Geropogon in Fl.Iran,1997;122.
22. Tahtadžjan E,Konspekt A,flory Kavkaza L Podospermumbicolor (Freyn&Sint.)Kuth .Conspectus Florae Caucasi,2008;3(1).
23.Tahtažan F A L Scorzonera bicolor.Flora Armenii,1995;9.
24.Grossgejm G A A.Flora kavkaza Scorzonera karjaginii,1934;4.
25. Karjagin H I Flora azerbajdžana,1961;8.
26. Boissier IEFlora Orientalis,1875;3.
27. Boissier J E.Scorzonera bulbipes.Flora Orientalis,1875;3.
28. Davis K P H.Flora of Turkey and the East Aegean Islands,1975;5.
29. Grossgejm LA A.Flora kavkaza,1934;4.
30. Güner A M (ed),Türkiye Bitkileri Listesi (Damarlý Bitkiler) ) 2012.
31. Komarov NFl.Scorzonera bicolor.URSS 29.1964
32.Mouterde O,Nouvelle P.Scorzonera bulbipes. flore du Liban et de la Syrie.Texte 3.1978-1984.
33.Tahtadžjan P A L.Scorzonera bicolor Freyn& Sint. Flora Armenii 9,1995.
34.Bahadır Acikara Ö,Hošek J,Babula P,Cvaˇcka J,et al.Turkish Scorzonera Species Extracts Attenuate Cytokine Secretion via Inhibition of NF-κB Activation,Showing Anti-Inflammatory Effect in Vitro.Molecules,2016;21(43).
35.International Conference of Plant Scientists.10th National Meeting of Plant Scientists.Pakistan,2008,April 21– 24.
36.MA,Sharifi Bigy S,Allahresani A,Malekaneh M.Assessment of Antioxidant Activity,Chemical Characterization and Evaluation of Fatty Acid Compositions of Scorzonera Paradoxa Fisch and C.A.Mey.Jundishapur J Nat Pharm Prod,2015;10(4):e19781.
37.Küpeli Akkol E,Bahadır Acıkara Ö,Süntar I,Burc¸Ergene I,et al.Ethnopharmacological evaluation of some Scorzonera species:In vivo anti-inflammatory and antinociceptive effects.Journal of Ethnopharmacology, 2012) ;140 :261–270.
38.Macleod G,AMES JM.Gas chromatography-Mass Spectroscopy of the Volatile Components of Cooked Scorzonera.Phytochemistry,1991;30( 3):883-888.
39.Encyclopædia Iranica.Isfahan, Pre-Islamic-Period.2006. Retrieved 31 December 2015.
40.Adeyinka,A,Liang H,Tina G.Removal of Metal Ion form Waste Water with Natural Waste.School of Engineering and Technology,2007;33:1-8.
41.AOAC (1996) method 981.12 Official Methods of Analysis of AOAC International.16 ed.,16.ed.Maryland:USA.
42.AOAC (2000) method 962.09,Official Methods of Analysis of AOAC International, 17th ed.14 ed., AOAC International,Gaithersburg:Maryland USA.
43.Ziarati P,Rabizadeh H.Safety and Nutritional Comparison of Fresh,Cooked and Frozen Mushroom(Agaricus bisporus).Intl J Farm & Alli Sci.,2013;2:1141-1147.
44.Ziarati P,Azizi N,Chemical Characteristics and Mineral Contents in Whole rice grains,Hulls,Brown rice,Bran and Polished Ali Kazemi Rice in Gilan province–North of Iran.Intl J Farm&Alli Sci,2013;2 :1203-1209.
45.Ziarati P,Rabizadeh H.The Effect of Thermal and Non Thermal of Food Processes and Cooking Method in Some Essential Mineral Contents in Mushroom (Agaricus bisporus) in Iran.J Nov.Appl Sci.,2013;2:954-959.
46.AOAC.Association of Official Analytical Chemists.Wet digestion for non–volatile metals in:AOAC official methods of analysis,1998;16th edition,4th revision,vol.1,chapter 9.
47.Ziarati P.Determination of Contaminants in Some Iranian Popular Herbal Medicines. J Environ Analytic Toxicol, 2012;2:120.doi:10.4172/2161-0525.1000120.
48.Ziarati P,Tosifi S.Comparing some physical and chemical properties of green olive (Olea europea L.) in Iran association with ecological conditions.IJPAES,2014;4:519-528.
49.ORA LABORATORY MANUAL FDA Office of Regulatory Affairs Office of Regulatory Science.Vol 6.DOCUMENT NO:IV-02,VERSION NO:1.5,EFFECTIVE DATE:10-01-03 REVISED:02-14-13.Available in Site:http://www.fda.gov/downloads/ScienceResearch/FieldScience/UCM092226.pdf
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/