J Sci Discov (2017); 1(1):jsd17003; DOI:10.24262/jsd.1.1.17003; Received April 20th, Accepted May 10th, Published May 20th.
Missing Dark in Modern Life Aids in Developing Breast Cancer
K.Pushkala1, P.D. Gupta2,3,*
1Director, Evening Stream, S.D.N.B. Vaishnav College for Women, Chennai, India 600 044.
2Adjunct Professor, Manipal University, Manipal, India, 576104.
3Adjunct Professor Gujarat University, Ahmedabad, India, 380001
* Correspondence: Dr. Gupta, Manipal University, Manipal, India; Tel: 09460892478, 09929370150; E-mail: pdg2000@hotmail.com.
Introduction
In modern industrialized societies cancer is fuelled by the excesses of modern life. Thanks to Thomas Alva Edison, who extended our working hours by putting light during dark hours. The melatonin hypothesis states that “the increased risk in BC (breast cancer) in recent decades in industrialised countries results in part from increased exposure to LAN” (Light at night) [1; 2;3]. Strong support for this hypothesis comes from the increased risk of BC in nightshift workers [4]. In a breakthrough study, while working with “born blind rats” we showed for the first time that “Estradiol is always inversely proportional to melatonin [5]. This led us to make many workable “Hypotheses”. The most important one is that “Blind woman has minimum risk of developing BC” [6-12]. We miss dark and suffer the consequences.
Three great scientists have to be remembered in the area of light, namely, Sir C V Raman, Prof. Albert Einstein and Thomas Alva Edison who revolutionized physics, chemistry and biology of light. Light is the only energy that exists in 2 forms viz., 1. Wave and 2. Particulate. This makes it to reach almost every place on the earth, since it is essential for life. But excess use of this energy during night LAN adversely affects human physiology and therefore is harmful.
Light and dark cycles – Essential for life
Ninety eight percent of sunlight enters through the eyes, and 2% through the skin. The 1,200 frequencies of light act on our body on an ongoing basis to power the cells, regulate biological clock and produce hormones, affect metabolism. When enough light is not there, all body functions start diminishing, and cause depression and adversely affect immune system. Earlier we studied the effects of day light in anopthalmic rats [5] and concluded that the mammalian eye sub serves at least two photic systems:
- A visual pathway, which enables us to see, is the upper pathway, which goes up to the occipital cortex which mediates the conscious perception of light and recognition of images, and a sub cortical system is a lower pathway goes to the hypothalamus that mediates light sensitive synchronization of the circadian pacemaker [5; 13; 14; 15].
- The lower pathway controls glands, regulates circadian rhythms, instructs the pineal gland to produce the appropriate hormones (serotonin during daylight; melatonin in the dark night) [16].Natural light has been used to treat depression, jaundice of neonates from times immemorial. However, now different intensities of artificial light are used to treat many diseases.With the advent of electricity, day light is extended and darkness is replaced by yellow incandescent light. The industrial revolution resulted in the fluorescent (“daytime” spectrum bulbs) replacing incandescent bulbs at homes and workspaces. Now fluorescent bulbs and screens are replaced by LED light.
Before the advent of artificial light our life (physiology) was synchronized with 12 hours day/12 hour night periods. The use of light at night to extend the day, dismissed natural life style which resulted in different disease. All these lighting systems predisposes us to more of blue light of 400-480nm in the electromagnetic spectrum inhibiting synthesis of melatonin, altering our circadian sleep and hunger rhythms, changes in alertness and possible psychological and disease-susceptibility disturbances. In the recent past these “blue light” effects have focused a significant research attention discussed as a public health issue [17].
Epidemiological studies suggest that chronic (not intermittent) exposure to light is associated with an increased risk of breast, prostate cancers especially hormone dependent cancers. Since melatonin is a master hormone and its production is hampered by continuous expose to light, regulation of gonadal hormones is also disturbs [5].
Topography, photoperiod and breast cancer
Major source of light on the earth is the sun. In the equatorial region, sun shines only for 12 hours and the rest 12 hours is the dark period, but on poles it is a different story. There are countries around the poles where night is missing. The sun shines continuously for 23/24 hrs. Therefore the physiology of the organisms which is aligned with light gets disturbed. The human settlements increased after the advent of artificial light in the zones where photoperiods are very much extended or reduced. In such regions, from the literature survey a several-fold difference in the incidence of breast cancer (BC) between countries closer to the poles than to the equator has been recorded (Table 1).
| S. No. | Name of the country | |||
| Towards poles |
Incidence rate /
10,0000 |
Towards Equator | Incidence rate / 10,0000 |
|
| 1 | Alaska | 125 | Magnolia | 7 |
| 2 | Belgium | 109.2 | Bhutan | 7 |
| 3 | Denmark | 101.1 | E Asia | 18 |
| 4 | France | 99.7 | S C Asia | 18 |
| 5 | Netherlands | 98.5 | Indonesia | 18.6 |
Note: Table showing the high incidence of BC in the countries closer to poles compared to equatorial region with disturbed circadian rhythm due to a long exposure of natural and /or artificial light.
Table 1. A comparison of incidence of BC in the populations living in countries closer to poles and equatorial regions respectively.
LAN on shift workers
The clue for the disturbance of circadian rhythm–cancer connection was first started from the study by Stevens on the effect of light and extremely low frequency electric and/or magnetic (ELF) fields on pineal melatonin production, and on the relationship of melatonin to mammary carcinogenesis (1). Artificial light at night disrupting the natural melatonin- estrogen balance was linked with the increase in hormone regulated BC among women [18-27]. Epidemiological studies have been carried out (15 studies including 8 cohort and 7 case-control studies) to understand the impact of night shift working system on the incidence of BC. The increased cancer risk has been reported in nurses, radio-telephone operators, flight attendants, and women employed in the enterprises, in which 60% of employees work at night [28]. A study involving members of the Californian Teachers found an increased risk of developing BC in women living in areas with the highest outdoor light at night, estimating the impact of indoor and outdoor light at night [29]. Risk of developing BC increased in women who worked night shifts for more than five years especially those who are regularly engaged in night work for at least four years prior to their first pregnancy, before their mammary systems had fully differentiated [30]. Earlier studies were done to understand how imbalance of natural melatonin- estrogen by artificial light at night could be linked with the increase in hormone regulated BC among women [31; 32; 33; 34; 35]. Therefore, it is not surprising that disruption of melatonin titer by circadian rhythms may be well associated with several diseases, particularly hormone regulated cancers such as breast and prostate cancers. Predictions were made long ago that women working a non-day shift would be at a higher risk of developing BC compared to the day-working women. Total revamping the shift work schedule taking all appropriate and effective measures against proven links to BC with health care system for women is still wanting worldwide. Proper education needs to be given on health activities, such as, campaigns to improve the understanding of the consequences and risks of night shift working pattern so as to minimize the development of BC [36]. Night shift working system has also been linked with prostate cancer in men similar to BC in women [37].
Blind women model to validate role of light in development of BC
In the survey, out of 2060 (collected during 2006 – 2013) menopausal blind women, we found only a few were suffering with breast cancer [7;8;9;10;12;38] gave a clue for the low prevalence of BC in blind menopausal women compared to sighted women in India [39] where stable circadian rhythm is maintained. This study was the first of its kind considering the influence of parameters such as total blindness / partial blindness /development of blindness before menarche /after menarche /development of blindness before pre-menopause/after menopause, on the prevalence of BC in blind women. Though earlier studies in Finland and Sweden [40-41] also reported low prevalence of BC in totally blind women compared to sighted women, the comparison between the incidences of BC between above mentioned parameters were wanting. Hahn indicated that, overall, women with bilateral blindness had almost half the risk of developing BC compared to sighted women from a US case control study [42]. An epidemiological survey showed thrice the elevated risk of developing BC, where age at onset of blindness has a profound role to play in the progression of BC. In this study postmenopausal stage of a woman were found to have more risk of developing BC than pre-menopausal stage [12]. Evans et al. [43] observed blind women with no perception of light (NPL) have a lower prevalence of BC compared to blind women with light perception (LP). But they observed little difference in these associations when restricted to postmenopausal women, non-shift workers or excluding women diagnosed with breast cancer within 2 or 4 years after onset of blindness.
Melatonin has been established to have anti-cancerous activity. From these studies it emerges that blind menopausal women may serve a model for understanding regulation of BC in relation to melatonin, light, estrogen and other hormones.
Action of light: Molecular Mechanisms
The external light sensitive organs in human are eyes, skin and internal organs such as suprachiasmatic nucleus and pineal are the ones take part in photoreactions in the body. When light is focused on the eye, various physical and chemical reactions are induced in the photoreceptor (specialized, light-sensitive cells) present on the retina by signal transduction pathway. Steven W. Lockley, and colleagues measured melatonin in 49 blind individuals and noted that melatonin cycles in blind are predictable [44]. “Light at night is now clearly a risk factor for BC. Blask et al. [45] explained that breast tumors are awake during the day, and melatonin puts them to sleep at night”. They also added that if artificial light is added to the night environment, the cancer cells become insomniacs. Further they showed in rat model, that an abnormal timing of melatonin peaks could have a powerful effect on cancer. Cancer causing chemicals were administered to rats and then over subsequent weeks, injected the animals daily with melatonin. The injections were timed to produce peaks during daylight hours, when melatonin concentrations should have been negligible. When injections were given in the mid-morning, tumors grew at the same rates seen in animals not receiving injections. However, in animals that received the hormone during the afternoon, an inhibitory effect of the hormone on tumor growth, not only in liver cancers, but also in BC was observed. Constant light, suppressed melatonin, increased cancer cell growth rates and increased Linoleic acid (LA) uptake into cancer cells. The opposite was seen in the light-dark group. The proposed mechanism is the suppression of nocturnal melatonin by exposure to LAN and subsequent lack of protection by melatonin on cancer cell receptor sites, which allows the uptake of LA, in turn enhances the growth of cancer cells. Melatonin is a protective oncostatic hormone and a strong antioxidant evolved in all plants and animals over the millennia. In vertebrates, melatonin is normally produced by the pineal gland during the early morning hours of darkness (even in nocturnal animals), and is suppressed by exposure to LAN. These findings suggest that there is a rhythm of sensitivity within tumor tissues or in cells susceptible to tumors formation and may be in people who can’t perceive light, the oscillating cycle of their biological clock causes their melatonin peaks to coincide with the inhibitory period of tumor cells more often than they do in light-sensitive people. Blask noted that in several laboratories working with cells and with tissues removed from animals indicates that a reduction of melatonin can alter the production of other hormones, may suppress the immune system’s ability to recognize and respond to newly emerging cancers, and appears to spur the growth of at least some tumor tissues [46]. Blue and green lights appear especially effective at inhibiting melatonin synthesis in healthy young men. For some colors, “17 lux was sufficient to produce strong melatonin suppression in these men and some had full suppression with exposure to as little as 5 lux, a little more illumination than what you’d have with full moonlight. His experiments with rodents proved that tumors can grow especially rapidly when exposed to constant light, presumably due to a near-total suppression of their melatonin levels in the microenvironment [45; 47].
Interaction between Pineal and Suprachiasmatic nuclei (SCN)
Human biological clock is set to be prevailing in SCN which produces Serotonin, the precursor of melatonin. The conversion of serotonin to melatonin is light dependent since, only in dark this reaction proceeds in foreword direction. The input pathways carrying photic and nonphotic information provided by the retina, hypothalamic SCN, paraventricular nuclei, preganglionic sympathetic regions of the spinal cord, superior cervical ganglion (SCG) are given to pineal gland which takes the responsibility to connect the endogenous clock to the environment. Since, the conversion of serotonin to melatonin takes place in dark only, blind women maintain a constant titer of the hormone unlike the sighted women. The circadian rhythm may be intact in blind women unlike in sighted women, however the photic receptors for image formation through light are non functional. The light dependent hormone is quantitatively more in blind women and therefore as it is mentioned can regulate lower levels of estrogen even in menopausal women.
Earlier we have shown that there is an inverse relationship between melatonin and estrogen [5], a cell proliferating hormone and a quantity of this can be a cause for the proliferation of breast cells. In menopause the control of estradiol and progesterone secretion is disturbed and therefore postmenopausal women are more vulnerable to BC.
Light as an epigenetic factor for activating cancer genes
The Greek word “epigenetics” implies features that are “in addition to” the traditionally inherited by genetic basis. In other words, a change in phenotype without a change in genotype — which in turn affects how cells read the genes. Epigenetic change is a regular and natural occurrence but can also be influenced by several factors including age, the environment/lifestyle, and disease state. The first human disease to be linked to epigenetics was cancer, in 1983 [48]. For the first time TakaIlilshi [49] isolated a piece of DNA in higher animals that controls the internal clock mechanism in the brain and eyes, regulating the daily ebb and flow of everything from sleep to moods to hormone levels as well. Scientists believe that this is one of several genes, perhaps 10 or so, that produce the proteins that keep the body running on schedule. The discovery of this gene was a “significant breakthrough” since this was first window into the molecular biology of biological clocks in mammals.
Out of many risk factors, which aids in development of BC, two very important epigenetic factors have been considered viz., Light and Food. Epidemiological evidence support the anticipation that light also could be a significant contributory factor for the higher incidence of BC in night shift workers due to extended day and low incidence in blind subjects due to absence of light through their eyes. Similarly some diets are better than others in the prevention of BC development. For prevention, not only individuals but governments should adopt certain measures for rotation of shift duties, commercial restriction on food additives; encourage people to eat less processed food and whole grains. It has been established that there is a close link between “Food and DNA” [8].
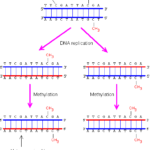
The best-known epigenetic marker is DNA methylation. One of the most fundamental questions in the control of gene expression in mammals is, how epigenetic methylation patterns of DNA and histones are established, erased, and recognized [50]. The initial finding of global hypomethylation of DNA in human tumors was soon followed by the identification of hypermethylated tumor-suppressor genes, and then, more recently the discovery of inactivation of microRNA (miRNA) genes by DNA methylation (Fig. 1).
Figure 1. Inheritance of the DNA methylation pattern. The DNA methyltransferases can methylate only the CG sequence paired with methylated CG. The CG sequence not paired with methylated CG will not be methylated. Hence, the original pattern can be.
DNA methylation occurs in a complex chromatin network and influenced by the modifications in histone structure, commonly disrupted in cancer cells [51;52].
The development of cancer has been associated with epigenetic alterations such as aberrant histone deacetylase (HDAC) activity. It was recently reported that valproic acid is an effective inhibitor of histone deacetylases and as such induces tumor cell differentiation, apoptosis, or growth arrest [53;54;55].
Expression of c-fos in SCN appears to be regulated by environmental light, and expresses protein which increase in intact brain by wide variety of stimuli including chemical and electrically induced seizures, noxious sensory stimulation, hormones, and water deprivation. The control of c-fos gene expression is complex and work in synchrony with other fos-like genes such as fra 1, fra 2 and fos B and jun genes such as c-jun, jun B and jun D. Several genes were discovered in recent years that are important to molecular components of the biological clock in mammals.
Due to post-translational modifications of the N-terminal tails of histone and methylation of cytosine residues in the DNA, Chromatin undergoes a variety of chemical modifications. Core histones are characterized by the presence of a histone fold domain and N-terminal tails of variable length that are subject of extensive post-translational modifications.
Histone modifications include: acetylation, methylation, phosphorylation, ubiquitylation, glycosylation, ADP-ribosylation and carbonylation. Many amino acids of histones are modified, including lysine residues that may be acetylated, methylated or coupled to ubiquitin; arginine residues may get methylated; and serine or threonine residues could be phosphorylated. Many of the modifications such as methylation of DNA and acetylation of histones can affect the other translation processes. They are positively or negatively correlated with specific transcriptional states or specific organization of repressive or open chromatin.
Histone methylation is a post-translational modification of histones which takes place on the side chains of both lysine (K) and arginine (R) residues. Histone methylation is a reversible process which is catalysed by histone methyltransferases (HMT), such as PRMT1 or Suv39H whereas histone demethylation is catalysed by histone demethylases, such as LSD1 or Jumanji domain-containing proteins. The regulational consequence of histone methylation on transcriptional state of a gene depends on the methylated residue and degree of methylation. Lysine can indeed be mono-, di- or tri-methylated.
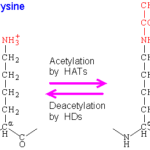
The modulation of chromatin condensation can be achieved via reversible acetylation on the lysine residues of histone tails. The acetylation reaction consists in the transfer of an acetyl group from acetyl coenzyme A (acetyl-coA) on the ε-amino group of the lysine residue, neutralizing the positive charge. This process results from a balance between the activities of two families of antagonistic enzymes: histone deacetylases (HDACs) and histone acetyltransferases (HATs), respectively removing or adding acetyl groups into core histone (Fig. 2).
Figure 2. Showing acetylation and deacetylation of Lysine
Histone phosphorylation occurs on serine and threonine residues and influences transcription, chromosome condensation, DNA repair and apoptosis. For example, phosphorylation of serine 10 and serine 28 on the tail of histone H3 (H3 phospho Ser10 or H3 phospho Ser 28) occurs early in mitosis when chromosome condensation is induced during S-phase. Mammalian DNA methylation is intricately connected to the presence of unmodified lysine 4 and methylated lysine 9 residues in histone H3. An interconnected network of methyl transferases, demethylases, and accessory proteins is responsible for changing or maintaining the modification status of specific regions of chromatin. The structural and functional interactions among members of this network are critical to processes that include imprinting and differentiation, deregulation of which is associated with disorders ranging from inflammation to cancer [56].
Recent molecular studies show that ER-negative breast cancer, results from the lack of ER gene transcription due to the methylation of the CpGisland 5′ to the gene. Because CpG island methylation is an early event in carcinogenesis and because methyl-deficient diets could not result in CpG island methylation, it is relevant to postulate that methyl-deficient diets may be a low risk factor for BC with methylated ER genes (as opposed to the disease with un methylated ER genes).
Breast cancer is one of the most dreadful diseases; nevertheless, by changing life style it can be prevented easily. The DNA-methylation and histone-modification patterns associated with the development and progression of cancer have potential clinical advantage. DNA hypermethylation markers are under study as complementary diagnostic tools, prognostic factors, and predictors of responses to treatment. For instance, the glutathione S-transferase gene (GSTP1) is hypermethylated in 80 to 90% of patients with prostate cancer, but it is not hyper methylated in benign hyperplastic prostate tissue. Analysis of hypermethylation of the CpGisland has potential diagnostic applicability for carriers of high penetrance mutations in tumour-suppressor genes. For example, identification of DNA hypermethylation in a breast-biopsy specimen from a carrier of a BRCA1 mutation could be useful when the pathological diagnosis is uncertain, because hypermethylation of the CpGisland is an early event in the development of cancer. Analysis of several hypermethylated genes detects twice as many tumour cells in breast ductal fluids as conventional cytologic analysis, and hypermethylated genes can be found in exfoliated cells at different stages in the development of cervical cancer. The application of DNA-hypermethylation markers as tumor markers in routine clinical practice will require rapid, quantitative, accurate, and cost-effective techniques and objective criteria for selection of the genes that are applicable to different tumor types.
Conclusions
Three different epidemiological survey results for BC incidence, namely, 1.blind menopausal women, 2. population on polar region, and 3. night shift workers univocally proved that more expose to light and no time to give chance for conversion of serotonin to melatonin which is a dark reaction raises incidence of BC. This may be due to lower levels of melatonin in the blood because serotonin is not able to get converted in to melatonin and whole hormonal milieu changes. Light induced BC is not genetic; they are mostly phenotypic. Epigenetics plays an important role in such cases. Prolonged exposure to light and missing dark periods not only changes melatonin levels in the body but may also bring about gene mutation and methylation which inturn change gene expression that may be responsible for higher incidence of (BC) cancers.
Competing interests
The authors declare that they have no competing interests.
References
1. Stevens RG. Am J Epidemiol. Electric power use and breast cancer: a hypothesis. 1987; 125:556-561.
2. Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. CA Cancer J Clin. Breast cancer and circadian disruption from electric lighting in the modern world. 2014; 64:207–18.
3. Yang WS, Deng Q, Fan WY, Wang WY, Wang X. Eur J Cancer Prev. Light exposure at night, sleep duration, melatonin, and breast cancer: A dose –response analysis of observational studies. 2014 ; 23: 269-276.
4. Swerdlow A. Sudbury, Health and Safety Excecutive. Research report: Shift work and breast cancer: a critical review of the epidemiological evidence.2003; 132; 1–28.
5. Jagota A, Olcese J, Harinarayana Rao S, Gupta PD. Brain Res. Pineal rhythms are synchronized to light-dark cycles in congenitally anophthalmic mutant rats. 1999; 825: 95-103.
6. Pushkala K, Gupta PD. Int J Med Med Sci. Prevalence of breast cancer in menopausal blind Women. 2009; 1: 425- 431.
7. Pushkala K, Gupta PD. Dark side of the night light Monograph. LAMBERT Academic Publishing, GmbH & Co. KG, Saarbrücken, Germany 2011; (123 Pages).
8. Gupta PD, Pushkala K. Prevention and Treatment of Breast Cancer by Light and Food In: Natural Products and their Active Compounds on Disease Prevention. Editors: Essa MM, Manickavasagan A, Sukumar E. Nova Science Publishers. C.G.C Press USA, 2012; 153-159.
9. Gupta PD, Usha N, Pushkala K. J Cell Tissue Res. Dark side of the night light: Implication in breast cancer. 2010; 10: 2173-84.
10. Pushkala K, Gupta PD. Int J Med Sciences and Biotechnology. Epigenetic Effect of Food for Cancer Management. 2013; 1: 1-11.
11. Pushkala K, Gupta PD.BAOJ Cancer Res Ther. Light and breast cancer: Is there any relationship?. 2016; 2: 12: 026.
12. Pushkala K, Gupta PD. Journal of Analytical Oncology. Increased Incidence of Breast Cancer Due to Long Exposure of Light.2016; 5: 146-152.
13. Foster RG, Provencio I, Hudson D, Fiske S, De Grip WJ, Menaker M. J Comp Physiol A. Circadian Photoreception in the retinally degenerate mouse (rd/rd). 1991; 169:39-50.
14. Chaurasia SS, Gupta PD. Curr Sci. Cryptochromes: The novel circadian photoreceptors. 1999; 77: 55.
15. Nagpal K, Vasavada AR, Gupta, P.D. (2005) Salient features of ocular biochemical status In: Concepts of Biochemistry for Medical students. Ed. Prof. L.M. Shrivastva, Senior Consultant & Head, Dept. of Biochemistry, Sir Ganga Ram Hospital, New Delhi. CBS Publishers & Distributors, 4596/1-A, 11Darya Ganj, New Delhi-110 002 (India). ISBN: 81-239-1182-3 (Reference book). (Pages-543-554).
16. Consumer Health Articles: LIGHT AND HEALTHwww.consumerhealth.org/articles/display.cfm?ID=…LIGHT AND HEALTH by:Olszewski, David, E.E., (from Health and Light by John Ott) …
17. Blue light has a dark side – Harvard Healthwww.health.harvard.edu/…/blue-light-has-a-dark-sideLight at night is bad for your health, and exposure to blue light emitted by electronics and energy … to the causation of cancer, diabetes, heart disease, …
18. Pukkala E, Auvinen A, Wahlberg G. BMJ. Incidence of cancer among Finnish airline cabin attendants 1967-92. 1995; 311: 649-652.
19. Tynes T, Hannevik M, Andersen A, Vistnes AI, Haldorsen T. Cancer Causes Control. Incidence of breast cancer in Norwegian female radio and telegraph operators. 1996; 7: 197–204.
20. Hansen J. J Natl Cancer Institute. Light at night, shiftwork, and breast cancer risk. 2001a; 93: 1513-1515.
21. Hansen J. Epidemiology. Increased breast cancer risk among women who work predominantly at night. 2001b; 12: 74-77.
22. Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, et al. Aviat Space Environ Med. Cancer incidence among 10,211 airline pilots: a Nordic study. 2003; 74: 699-706.
23. Hansen J. Cancer Causes Control. Risk of breast cancer after night- and shift work: current evidence and ongoing studies in Denmark. 2006; 17: 531-537.
24. Davis S, Mirick DK, Stevens RG. J Natl Cancer Inst. Night shift work, light at night and risk of breast cancer. 2001; 93: 1557–1562.
25. Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Epidemiology. Night work and risk of breast cancer. 2006; 17: 108-111.
26. Franzese E, Nigri G. Prof Inferm. Night work as a possible risk factor for breast cancer in nurses. Correlation between the onset of tumors and alterations in blood melatonin levels.2007; 60:89-93.
27. Viswanathan AN, Hankinson SE, Schernhammer ES. Cancer Res. Night shift work and the risk of endometrial cancer. 2007; 67: 10618-10622.
28. Brudnowska J, Pepłońska B. Med Pr. Nightshift work and cancer risk: a literature review.2011; 62:323-338.
29. Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L. Reynolds P. Epidemiology. Light at night and breast cancer risk among California teachers.2014; 25:697-06.
30. Menegaux F, Truong T, Anger A, Cordina-Duverger E, Lamkarkach F, Arveux P, Kerbrat P et al. Int J Cancer. Night work and breast cancer: A population-based case- control study in France (the CECILE study). 2013; 13: 924-31.
31. Tynes T, Hannevik M, Andersen A, Vistnes AI, Haldorsen T. Cancer Causes Control. Incidence of breast cancer in Norwegian female radio and telegraph operators. 1996; 7: 197–204.
32. Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, et al. Aviat Space Environ Med. Cancer incidence among 10,211 airline pilots: a Nordic study. 2003; 74: 699-706.
33. Franzese E, Nigri G. Prof Inferm. Night work as a possible risk factor for breast cancer in nurses. Correlation between the onset of tumors and alterations in blood melatonin levels.2007; 60:89-93.
34. Viswanathan AN, Hankinson SE, Schernhammer ES. Cancer Res. Night shift work and the risk of endometrial cancer. 2007; 67: 10618-10622.
35. Hansen J. J Natl Cancer Inst. Light at Night, Shift work, and Breast Cancer Risk. 2001; 93: 1513-15.
36. Moukangoe PIJ, Jansen van Rensburg, MS. Open Journal of Epidemiology. The Association of Night Shift Work with the Development of Breast Cancer in Women.2015; 5: 14-21.
37. Brudnowska J, Pepłońska B. Med Pr. Night shift work and cancer risk: a literature review.2011; 62:323-338.
38. Mettlin C. A Can J Clin. Global breast cancer mortality statistics. 1999: 49:138–144.
39. Shantha V, Swaminathan R, Balasubramanian, S. (2008). Cancer incidence and mortality in Chennai-India: 2003-2005. National cancer registry Programme Cancer Institute (W.I.A.), Chennai.
40. Verkasalo PK, Pukkala E, Stevens RG, Ojamo M, Rudanko, SL. Brit. J. Cancer. Inverse association between breast cancer incidence and degree of visual impairment in Finland.1999; 80:1459–1460.
41. Feychting M, Osterlund B, Ahlbom, A. Epidemiology. Reduced cancer incidence among the blind. 1998; 9: 490-494.
42. Hahn RA. Epidemiol. Profound bilateral blindness and the incidence of breast cancer. 1991; 2 : 208–210.
43. Evans F, Erin E, Stevens RG. Homayoun T. Schernhammer ES et al. Cancer Causes & Control.Total visual blindness is protective against breast cancer. 2009;20: 1753-1756.
44. Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. J Clin Endocrinol Metab. Relationship between melatonin rhythms and visual loss in the blind. 1997;82 :3763-3770.
45. Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA et al. Cancer Res. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. 2005; 65:11174-11184.
46. Carillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ.A Endocrine. Review of the multiple actions of melatonin on the immune system. 2005; 27:189–200.
47. Blask DE, Dauchy RT, Brainard GC, Hanifin JP. Integr Cancer Ther. Circadian Stage-Dependent Inhibition of Human Breast Cancer Metabolism and Growth by the Nocturnal Melatonin Signal: Consequences of Its Disruption by Light at Night in Rats and Women. 2009; 8: 341-353.
48. A.P. Feinberg, B. Vogelstein. Nature. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. 1983; 301: 89–92.
49. Takahashi T, Kaneko M, Mori Y, Tsuji M, Kikuchi N, Hiramune T. J VetMed Sci. Phylogenetic analyses of Staphylococcus based on the 16s r DNAsequence and assignment of clinical isolates from animals.1997; 59: 775-783.
50. Esteller M. N Engl J Med. Epigenetics in Cancer.2008; 358, 1148-1159.
51. Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA. Cancer Cell. Specific activation of micro RNA-127 with down regulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. 2006; 9: 435-443.
52. Kouzarides T. Cell. Chromatin modifications and their function. 2007; 128: 693-705.
53. Verma M, Maruvada P, Srivastava S. Crit Rev Clin Lab Sci. Epigenetics and cancer. 2004; 41: 585-607.
54. Rountree MR, Bachman KE, Baylin SB Nature Genet. DNMT1 binds HDAC2 and anew co-repressor, DMAP1, to form a complex at replication foci. 2000; 25: 269-277.
55. Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN,Bird A. Nature. Transcriptional repression by the methyl-CpG-bindingprotein MeCP2 involves a histone deacetylase complex. 1998; 393: 386-389.
56. Cheng X, Blumenthal RM. Biochemistry. Coordinated chromatin control: structural and functional linkage of DNA and histone methylation. 2010; 49: 2999-3008.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/