J Med Discov (2024); 9(2): jmd24072; DOI:10.24262/jmd.9.2.24072; Received May 10th, 2024, Revised May 29th, 2024, Accepted June 18th, 2024, Published June 24th, 2024
The study on differences and individual heterogeneity in immune regulation between Human umbilical cord mesenchymal stem cells and their exosomes
Tianyun Wu1, Juan Xia2,Miao Xuan2,Li Zhang2, Lei Chen2
1Key Laboratory of Spine and Spinal Cord Injury Repair and Regeneration (Tongji University), Shanghai Tongji Hospital, Tongji University of Medicine, Shanghai 200065, China
2Clincal research center,Shanghai Tongji Hospital, Tongji University of Medicine, Shanghai 200065, China
* Correspondence:Lei Chen,Clincal research center,Shanghai Tongji Hospital, Tongji University of Medicine, Shanghai 200065, China. Email:chenlei880109@126.com
Abstract
The differences of immunomodulatory capability between human umbilical cord mesenchymal stem cells (hucMSCs) from different donor sources and their exosomes were studied in this paper. Five hucMSC donors were randomly selected and cultured to the 5th passage to detect their surface markers, morphology, phenotype and differentiation abilities. The supernatants of P5 hucMSCs from different donors were collected to extract human cord mesenchymal stem cell exosomes (hucMSCs-ex). In the experimental groups, peripheral blood mononuclear cells (PBMCs) were co-cultured with hucMSCs or their corresponding hucMSCs-ex. Flow cytometry was used to detect the inhibitory effect of hucMSCs and hucMSCs-ex on CD3+ cells, Th1/Th2/Th17 cell subsets and TNF-α secretion and the promoting effect on Treg cell subsets. The results showed that different donor-derived P5 hucMSCs had heterogeneous inhibitory effects on CD3+ cell proliferation, Th1/Th17 cell subsets proliferation and heterogeneous promotion effects on Treg cell subsets but had homogeneous inhibitory effects on TNF-α secretion. P5 hucMSCs-exs did not inhibit the proliferation of CD3+ cells, but increased the apoptosis of CD4+ cells. HucMSCs-ex had no inhibitory effect on Th1 cell subsets, but increased the proportion of Th2 cell subsets. hucMSCs-ex had heterogenous inhibitory effects on the proliferation of Th17 cell subsets and the secretion of TNF-α, but have homogenous promotion effects on Treg cell subsets. P5 hucMSCs had stronger immune regulation capacity than their hucMSCs-ex. However, compared with the hucMSCs groups, the hucMSCs-ex groups had more homogeneous immunomodulatory capacity on inhabiting Th17 cell subsets proliferation and promoting Treg cell subsets. Since P5 hucMSCs and hucMSCs-ex from different donors have different immunomodulatory capabilities, hucMSCs and hucMSCs-ex should be screened before clinical application to ensure the effectiveness of immunomodulatory capabilities.
Keywords:Umbilical cord mesenchymal stem cells, Exosomes, Immunomodulatory Capacity, Individual Heterogeneity
Introduction
Mesenchymal stromal cells (MSCs) have been increasingly used in clinical practice as a new treatment method since MSCs have the ability to differentiate to osteoblasts, adipocytes and chondroblasts[1]. In addition, mesenchymal stem cells have immunomodulatory properties and are suitable for tissue repair and treatment of various diseases, such as rheumatoid arthritis (RA) [2], diabetes (DM) [3], and myocardial infarction (MI) [4] etc. Human umbilical cord mesenchymal stem cells (hucMSCs) are potential application cells for cell therapy, because compared with stem cells from other sources, hucMSCs have the advantages of easier collection, lower ethical risk and lower immunogenicity[5]. However, pulmonary obstruction effect is the major obstacle for intravenous (IV) stem cell delivery, which means most of administered stem cells are initially trapped in the lungs [6]. The main pathway for MSCs to exert therapeutic effects is to secrete extracellular vesicles (EV), such as exosomes (EXO), membrane-derived vesicles (MV) and apoptotic bodies to mediate cell communication between stem cells and recipient target cells [7]. Exosomes, the most important paracrine products of MSCs, can act as a vehicle to transport biologically active ingredients to the surrounding cells and circulatory system, and even cross the blood-brain barrier [8]. Human umbilical cord mesenchymal stem cell exosomes (hucMSCs-ex) have clinical application potential, especially in cell-free therapy. Exosomes are 40 to 160 nm membrane microvesicles, containing important biologically active ingredients, with surface markers of tetratransmembrane proteins (CD9, CD63 and CD81), Alix, and Tsg101 [9]. As nano-sized vesicles of endocytic origin, exosomes have intrinsic ability to cross biologic barrier. Exosomes can transfer the biological information content of the parent cell to target cells through endocytosis, direct fusion with the cell membrane of the receptor, or interaction between the ligand and the receptor [10]. In addition, if exosomes are purified from a compatible cell source, they are immunologically inert [11]. Li proved that hucMSCs-ex was well tolerated in animal models, and there was no systemic allergic reaction after transplantation of heterogeneous exosomes into guinea pigs [12]. Compared with hucMSC-based therapy, hucMSCs-ex has the advantages of non-tumorization, easier preservation and transplantation, low immunogenicity, and no involvement in cell apoptosis [13], leading a promising clinical application. However, whether hucMSCs-ex has the same immunomodulatory effect as hucMSCs needs to be supported by experimental data. In this study, we compared the immunomodulatory capacity of hucMSCs and hucMSCs-ex on PBMC.
Materials and methods
hucMSCs isolation and culture
Strict screenings were subdivided into no family genetic history, no history of infectious diseases, and non-carrying of nine human-derived viruses, including HBV, HCV, HIV, syphilis, CMV, EMV, HHV6, HHV7, HTLV, Human parvovirus). Puerpera who tested negative signed an informed consent form for voluntary donation of umbilical cord. The umbilical cords of healthy term cesarean birth newborns were collected by laboratory personnel under aseptic conditions. The combined enzyme digestion method was adopted for the primary isolation of human umbilical cord mesenchymal stem cells [14]. Then, hucMSCs were cultured to the fifth passage by using α-MEM medium (Hyclone, Logan, Utah, USA) added with serum replacement (Ultra GroTM AventaCell BioMedical, USA). The quality of P5 hucMSCs from different donors was tested according to GMP standards.
hucMSCs identification and potential differentiation
The morphology of P5 hucMSCs was observed by inverted microscope (Nikon, Japan). The surface markers of P5 hucMSCs, including CD 90, CD 44, CD 105, CD 73, CD34, CD45, CD19, CD11b, HLA-DR, were labeled by using Human MSC Analysis Kit antibody kit (BD Biosciences,Franklin Lakes,NJ,USA), and then were analyzed by BD C6 flow cytometry [15]. P5 hucMSCs were plated into 24-well plates at a density of 2×104 per well. After 48h, the culture medium was changed to osteogenesis, adipogenesis and chondrogenesis differentiation medium (Gibco, Grand Island, NY, USA). Chondrogenic differentiation was detected through Alcian Blue staining medthod after 14 days; Adipogenic differentiation was detected through Oil Red staining method after 21 days; Osteogenic differentiation was detected through Alizarin Red S staining method after 21 days. The differentiation ability of hucMSCs was observed by microscopy [16].
Isolation and identification of hucMSCs-ex
Culture supernatant was collected when the confluence of P5 hucMSCs reached 90%. hucMSCs-ex were collected by differential centrifugation [17], and the precipitated exosomes were resuspended in 150 μL PBS. The concentration of exsomal protein was detected by Micro BCA Protein Assay Kit (Sangong Biotech, Shanghai, China). The test was repeated 3 times and the average value of exsomal protein concentration was taken. Exosomes were evaluated by scanning electron microscopy (SEM), nanoparticle tracking analysis and Western Blotting [18]. The particle size of hucMSCs-ex was detected by ZETA VIEW (Particle Metrix, Germany) and the required data was collected by the relevant parameters of the software. Markers, including CD6、CD63、Alix、and ISG101 (Solarbio, China) were identified by Western Blotting.
Co-cultivation of PBMCs and hucMSCs or hucMSCs-ex
After resuscitation, PBMCs were divided into control groups, hucMSCs experimental groups and hucMSCs-ex experimental groups. Three replicate wells were set up in each group. The control groups were PBMCs cultured individually. Five P5 hucMSCs strains from different donor sources were randomly selected as the hucMSCs experimental groups, numbered as hucMSC-1, hucMSC-2, hucMSC-3, hucMSC-4, hucMSC-5. HucMSCs were resuscitated 24 hours in advance, and inactivated by adding mitomycin C (Abmole, USA) at a final concentration of 10 μg/mL for 30 minutes, and then washed twice with PBS. HucMSCs were plated in 12-well plates (Costar, Corning, NY) and cultured with RPMI 1640 medium (Corning, NY, USA) containing 10% FBS (Corning NY, USA). The P5 hucMSCs and PBMCs were co-cultured at a ratio of 1:5. In the previous co-cultivation protocol of the exosomes experimental group, there are two experimental designs, one is based on the concentration of exosomes, and the other is the number of exosomes particles. Chen et al. tested the effect of different concentrations of MSCs-derived exosomes (0, 5, 10, and 20 μg) on the regulation of PBMC, and the results showed that the experimental group of 20 μg MSCs-derived exosomes had the best immunomodulatory effect [19]. According to the experimental design of Del Fattore et al., 4.6×108 exosomal particles were added to verify the immunoregulatory effect of MSCs-derived extracelluar vesicles on T Lymphocytes [20]. In our experiment, the concentration of hucMSCs-derived exosomes added to each well was 20μg, which was converted into a particle number of 6.84×108. PBMCs were plated in 24-well plates with a quantity of 1.0×106/well. The P5 hucMSCs-ex experimental groups were assigned the corresponding numbers hucMSCs-ex-1, hucMSCs-ex-2, hucMSCs-ex-3, hucMSCs-ex-4, hucMSCs-ex-5.
Immunomodulation assays of PBMCs
To evaluate the proliferation of PBMCs, the cells were pre-labeled with 0.5μM 5-carboxyfluorescein diacetate succinimidyl ester (CFSE, Cell TraceTM, Thermo Fisher Scientific, Waltham, Massachusetts, USA). Lineage-driving cytokine, including anti-human CD3/CD28 antibody (1μg/mL), IL-2 (10ng/mL) and Penicillin/Streptomycin (1%) were added to the cell culture system. After 72 hours of culture, the suspended PBMCs were collected, and the proliferation rate of lymphocytes was detected by flow cytometry. For the detection of CD3+ cells and CD4+ cell subpopulation, the following monoclonal antibodies were used: BV421-CD3, PE-CD4, Fixed Viability Stain 780 (BD Biosciences), APC-Annexin V (Biolegend) and CFSE-APC (Cell TraceTM). At the same time, the cell supernatant was collected at the 72h time point, and the TNF-α secretion (pg/mL) was detected by using Th1/Th2/Th17 CBA flow cytometry kit antibody (BD Biosciences, New Jersey, USA).
Immunomodulation assays of Th1/Th2/Th17 cell subsets
To evalute the proliferation of Th1/Th2/Th17 cell subsets, lineage-driving cytokine, including anti-human CD3/CD28 antibody (1μg/mL) and Penicillin/Streptomycin (1%), were added to the cell culture systems [21]. After 43 hours of culture, PBMCs were stimulated with leukocyte activation cocktail, by adding GolgiStop and GolgiPlug (BD Biosciences, Franklin Lakes, NJ, USA). The new mixed cell system was incubated at 37℃and 5% CO2 for 5h. Then the suspended PBMCs were collected, and the proliferation rate of Th1/Th2/Th17 cell subsets were detected by flow cytometry. For detection of Th cell subsets in CD4+ helper T lymphocytes, the following monoclonal antibodies were used: FITC-CD3, PE-Cy7-CD4, BV421-IL-4, PE-IL-17A, Fixable Viability Stain 780 (BD Biosciences, New Jersey, USA) and AF647-IFN-γ (Biolegend, California, USA).
Immunomodulation assays of Treg cell subsets
To evalute the proliferation of Treg cell subsets, lineage-driving cytokine, including anti-human CD3/CD28 antibody (1μg/mL), IL-2 (20ng/mL), IL-15 (20ng/mL) and Penicillin /Streptomycin (1%) was added in the cell culture system [22]. After culturing for 120h at 37℃contaning 5% CO2, the suspended PBMCs were collected. CD4+CD25highFoxp3+ cells were considered regulatory T cells. For Treg cell subset, the following monoclonal antibodies were used: FITC-CD3, V500-CD4, BV421-CD25, PE-Foxp3 and Fixable Viability Stain 78 0(BD Biosciences, New Jersey, USA) To analyze the Foxp3+ expressed in CD4+ cells, Foxp3/Transcription Factor Staining Buffer Set (eBioscience, Thermo Fisher Scientific, Waltham, Massachusetts, USA) were used.
Flow cytometry analysis
All antibodies were purchased from BD and Biolegend. The PBMCs of the control groups and the experimental groups were collected from the culture plate and centrifuged at 1500 rpm for 5 minutes. The cell pellet was resuspended in PBS/FBS (2%), and the single cell suspension was incubated with the directly conjugated antibody for 30 minutes at 4℃ in the dark. After labeling, the cells were washed once in PBS and resuspended in Paraformaldehyde (4%). Data were acquired with BD LSRFortessa, and at least 100,000 events were collected for each dataset.
Statistical analysis
The software package Flowjo V10 was used for analyzing data of BD LSRFortessa. The experimental data was obtained through three independent repeated experiments and GraphPad Prism 5.0 was used for statistical analysis and statistical drawing. The statistical results are expressed as mean ± standard deviation. The quantitative data were compared by one-way ANOVA (S-NK). P-value <0.05 means that the difference is statistically significant.
Results
The culture and identification of MSCs
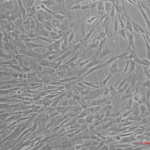
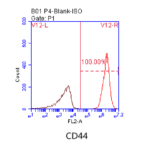
The morphology of P5 hucMSCs showed spindle shape (Fig.1). The results of flow cytometry showed that hucMSCs lacked the hematopoietic markers, such as CD34–, CD45–, CD19–, CD11b–, HLA-DR–, while expressed positive mesenchymal markers, such as CD90+, CD44+, CD105+, CD73+ (Fig.2). After culturing in osteogenic induction medium for 21 days, hucMSCs showed positive lipid droplets with Oil Red O staining (Fig.3A). After culturing in osteogenic induction medium for 21 days, hucMSCs showed calcium deposits with Alizarin Red staining (Fig.3B). After culturing in chondrogenic induction medium for 14 days, hUC-MSCs showed differentiating into chondroblast in vitro with Alcian Blue staining (Fig.3C).
Figure.1 Cell morphology of P5 hucMSCs
Figure.2 Cell phenotype of P5 hucMSCs
Figure.3 Multiple differentiation potentials of P5 hucMSCs. From left to right, multilineage differentiation potentials of hucMSCs for adipogenesis, osteogenesis and cartilage are shown.
The isolation and identification of exsome
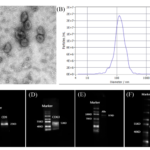
The isolated hucMSCs-ex were evaluated by scanning electron microscopy (SEM) , nanoparticle tracking analysis (ZETA VIEW) and Western Blotting to ensure that high-quality exosomes were obtained. Scanning electron microscopy showed circular membrane vesicles (Fig.4A). hucMSCs-ex showed an average size of 120.7 nm in diameter (Figure 4B), which was detected by ZETA VIEW. hucMSCs-ex had immune responses to key exsomal membrane proteins, such as CD9, CD63, Alix, ISG101, etc. (Fig.4C-4F).
Figure.4 Identification of exosomes. (A) Scanning electron microscopy was used to examine the structure of exosome. Bar 100nm. (B) ZETA VIEW was used to detect particle size of exosome (C-F) Key exosomal membrane proteins were analyzed by Western-Blot, expressing CD9, CD63, Alix, ISG101.
Effects of hucMSCs and hucMSCs-ex on immunomodulation of PBMCs
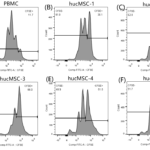
Anti-CD3/CD28 co-stimulation in 72h cultures significantly increased the number of proliferating CD3+ cells in the control group, showing continuous proliferation peaks (Fig.5A), while 72h co-culture with hucMSCs inhibited the proliferation peak of CD3+ cells. Significant effects were observed on the proliferation of CD3+ cells (88.70±0.80% vs 59.67±2.07%, 60.43±1.60%, 34.30±0.65%, 47.93±0.90%, 51.70±4.80%, P<0.001) (Fig.5B-5F). Comparison between groups showed that the five strains of hucMSCs had heterogeneous inhibitory abilities on CD3+ cells (Fig.7A). HucMSC-3 strain had the strongest capability to inhibit the proliferation of CD3+ cells, with an inhibition rate of 61.97%, while hucMSC-2 strain had the weakest inhibitory ability, with an inhibition rate of 32.46%.
The hucMSCs-ex experimental groups showed no inhibitory effect on the proliferation of CD3+ cells by fluorescence analysis of CFSE (91.30±0.78% vs 90.00±1.35%, 90.70±1.77%, 91.20±0.79%, 90.03±1.11%, 90.17±0.59%, P>0.05) (Fig.6A-6F) and there was no significant difference between the groups (P>0.05) (Fig.7B). However, the hucMSCs-ex experimental groups could significantly increase apoptosis of CD4+ T cells subsets (1.08 ± 0.11% vs 1.44± 0.05%, 1.46 ± 0.11%, 1.55 ± 0.10%, 1.70 ± 0.03%, 1.92 ± 0.12%, P <0.01).
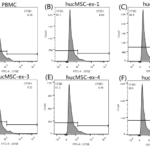
Figure.5 Proliferation ratio of CD3+ in different hucMSC experimental groups after 72h culture (A~F): (A) The proliferation rate of CD3+ cells in control group was 88.3%. (B) The proliferation rate of CD3+ cells in hucMSC-1 group was 61.9%. (C) The proliferation rate of CD3+ cells in hucMSC-2 group was 62.0%. (D) The proliferation rate of CD3+ cells in hucMSC-3 group was 34.0%. (E) The proliferation rate of CD3+ cells in hucMSC-4 group was 48.5%. (F) The proliferation rate of CD3+ cells in hucMSC-5 group was 51.7%. The proliferation ratio of the PBMCs control group (cultured individually) and the hucMSCs experimental groups (PBMCs co-cultured with P5 hucMSCs) was determined by flow cytometry. After 72 h of culture under the same stimulation conditions, the proliferation ratio of CD3+ cells was significantly higher in the control group than in the experimental groups.
Figure 6 Proliferation ratio of CD3+ in different hucMSCs-ex experimental groups after 72 h culture (A~F): (A) The proliferation rate of CD3+ cells in control group was 91.6%. (B) The proliferation rate of CD3+ cells in hucMSC-ex-1 group was 91.1%. (C) The proliferation rate of CD3+ cells in hucMSC-ex-2 group was 90.4%. (D) The proliferation rate of CD3+ cells in hucMSC-ex-3 group was 91.8%. (E) The proliferation rate of CD3+ cells in hucMSC-ex-4 group was 91.2%. (F) The proliferation rate of CD3+ cells in hucMSC-ex-5 group was 89.5%. The proliferation ratio of the PBMCs control group (cultured individually) and the hucMSCs-ex experimental groups (adding different strains of exosomes) was determined by flow cytometry. After 72h of culture under the same stimulation conditions, there was no inhibitory effect on the proliferation of CD3+ cells.
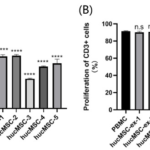
Figure.7 Regulation of CD3+ cells by P5 hucMSCs and hucMSCs-ex (A) Statistical analysis of the proliferation ratios among hucMSC experimental groups showed that all five hucMSC strains had significantly inhibiting effect on the proliferation of CD3+ cells (P<0.001) and differences between groups were statistically significant. HucMSC-3 strain had the strongest inhibitory effect, with the inhibition rate of 61.97%. (B) Statistical analysis of the proliferation ratio among hucMSCs-ex experimental groups showed all five hucMSCs-ex had no inhibitory effect on the proliferation of CD3+ cells and no difference between groups (P> 0.05).
The effect of hucMSCs and hucMSCs-ex on immunomodulation of Th cell subsets
The proportion of Th cell subsets in CD4+ cells in the control groups and the experimental groups was detected by flow cytometry. Except for the hucMSC-4 strain (32.50±1.50% vs 30.80±1.32%, P>0.05), the other 4 strains of hucMSCs could inhibit the proliferation of Th1 cell subsets, and the inhibitory effects were extremely significant (32.50±1.50% vs 23.40±0.85%, 21.77±0.95%, 23.43±0.31%, 24.47±0.74%, P<0.001) (Fig8A). Comparison between groups showed that the five strains of hucMSCs had heterogeneous inhibitory abilities on Th1 cell subsets. HucMSC-2 strain had the strongest capability to inhibit the proliferation of Th1 cell subsets, with an inhibition rate of 32.95%, while hucMSC-4 strain had no inhibitory effect, with an inhibition rate of 5.21%. However, five strains of hucMSCs-ex had no inhibitory effect on Th1 cell subsets (28.07±1.19% vs 28.40±1.42%, 26.43±0.34%, 29.60±1.44%, 28.57±0.52%, 29.33±0.31%, P>0.05) (Fig8B) and there was no significant difference between groups (P>0.05). However, the hucMSCs-ex experimental groups could promote the proliferation of Th2 cell subsets, and had significant effects (1.56±0.12% vs 2.63±0.23%, 2.85±0.15%,.2.21±0.09%, 2.33±0.16%, 2.24±0.08%, P <0.01)。
All five strains of hucMSCs had significant inhibitory effects on Th17 cell subsets (6.14 ± 0.22% vs 4.25± 0.04%, 2.88 ± 0.16%, 3.01 ± 0.22%, 2.50 ± 0.15%, 2.39 ± 0.07%, P>0.01) (Fig8C), and the inhibitory effects were different between groups. HucMSC-5 strain had the strongest capability to inhibit the proliferation of Th17 cell subsets, with an inhibition rate of 61.10%, while hucMSC-4 strain had the weakest inhibitory effect, with an inhibition rate of 30.62%. The five strains of hucMSCs-ex also had significant inhibitory effects on Th17 cell subsets (5.17±0.32% vs 4.17± 0.21%, 4.10±0.07%, 4.34±0.08%, 4.20±0.17%, 4.33±0.12%, P <0.05) but there was no difference between groups (P>0.05) (Fig8D).
The proliferation ratio of Treg (CD4+CD25highFoxp3+) in CD4+helper T lymphocytes was detected by flow cytometry. Compared with the control group of PBMCs cultured individually, the five experimental groups of hucMSCs from different donors had significant promotion effects on Treg cell subsets (7.43±0.03% vs 9.97±0.05%, 10.05±0.01%, 12.53±0.13%, 12.93±0.22%, 10.87 ± 0.75%, P<0.01) (Fig8E), and the promotion rates were different between groups. HucMSC-4 strain had the strongest capability to promote the proliferation of Treg cells, with an promoting ratio of 74.13%, while hucMSC-1 strain had the weakest promotion effect, with an promoting ratio of 34.27%. The hucMSCs-ex experimental groups could also promote the proliferation of Treg cell subsets, and the promotion effects were extremely significant (2.90±0.05%, 3.60±0.01%, 3.71±0.11%, 3.60±0.10%, 3.74±0.12%, 3.73±0.10%, P<0.01) (Fig8F). The results of comparison between groups showed that there was no difference between groups (P>0.05). HucMSC-3 strain had the strongest capability to promote the proliferation of Treg cells, with an promoting ratio of 29.44%.
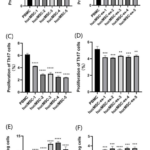
Figure.8 Regulation of T lymphocytes subsets by P5 hucMSCs and hucMSCs-ex. Flow cytometry was used to detect (A~B) Th1 cell subsets (CD4+/IFN-γ+), (C~D) Th17 cell subsets (CD4+/IL-17A+) and (E~F) Treg cell subsets (CD4+/CD25 high/Foxp3+) in CD4+ T lymphocytes /FoxP3+) in PBMCs control group, P5 hucMSCs experimental groups and hucMSCs-ex experimental groups. (A) The results showed that except the hucMSC-4 strain, the other four strains of hucMSCs from different sources could inhibit Th1 cell subsets, and the inhibition effects were extremely significant (P<0.001). (B) The hucMSCs-ex had no inhibition effect on Th1 cell subsets, and there was no significant difference between groups (P>0.05). (C) The results showed that all five hucMSCs strains inhibited the proliferation of a subset of Th17 cells with extremely significant effects (P<0.001). The inhibition effect showed differences among groups, where the hucMSC-5 strain had the strongest inhibition effect with an inhibition rate of 61.10%, and the weakest inhibition effect was the hucMSC-1 strain, with an inhibition rate of 30.62%. (D) The results showed that the hucMSCs-ex had significant inhibitory effect on Th17 cell subsets (P<0.01) and there was no difference between the groups (P>0.05). (E) The results showed that five strains of hucMSCs from different sources could promote the proliferation of Treg cell subsets, and the effect was extremely significant (P<0.001). There were significant differences in promoting effects, and hucMSC-4 had the highest promoting rate, which was 74.13%. (F) The results showed that five strains of hucMSCs-ex from could promote the proliferation of Treg cell subsets, and the effect was extremely significant (P<0.01). There were significant differences in promoting effects, and hucMSC-4 had the highest promoting rate, which was 29.44%
The effect of hucMSCs and its derived exosome on proliferation of TNF-α
After co-cultured for 72h, the cell supernatant of PBMCs was collected and incubated with BD™ Cytometric Bead Array (CBA) Human Th1/Th2/Th17 CBA Kit to detect the secretion of TFN-α (pg/mL).Compared with the PBMCs cultured alone in the control group, the results of the hucMSCs experimental group showed that the concentration of TNF-α was reduced, and the inhibitory effect was extremely significant (191.30±22.66 vs 14.50±1.40, 17.43±3.64, 14.50±1.95, 17.43±1.94, 16.38±2.75, P<0.001 ) (Fig9A). There was no difference in the inhibition of TNF-α secretion by five strains of hucMSCs (P>0.05), and the inhibition rate reached more than 90%. The hucMSCs-ex experimental groups also had inhibitory effects on the secretion of TNF-α, and the inhibitory effects were significant (427.44±6.48 vs 342.38±5.98, 272.58±31.20, 347.41±10.03, 270.25±18.01, 263.52±37.20, P<0.05) (Fig9B). There were differences among the groups, and the inhibition rate ranged from 18.72% to 38.35%.
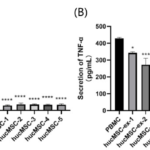
Figure.9 Regulation of TNF-α secretion by P5 hucMSCs and hucMSCs-ex (A) The results showed that the inhibitory effects of the five hucMSC strains on the secretion of TNF-α were extremely significant (P<0.001), and the inhibition rate were all over 90%. There was no difference in the inhibitory effect of five hucMSCs strains on TNF-α secretion among the groups (P>0.05). (B) The results of the hucMSCs-ex experimental groups showed that exosomes of P5 hucMSCs had inhibitory effects on the secretion of TNF-α, and the inhibitory effect was significant (P<0.05). There were differences between the groups.
Discussion
MSCs have the dual advantages of regulating inflammation and tissue remodeling, making stem cell therapy a hot spot in current treatment research [23]. The immunomodulatory properties of MSCs mainly depend on cell-to-cell signal transduction and paracrine signal transduction. The immunomodulatory properties of MSCs mainly depend on intercellular signal transduction and paracrine signal transduction [24]. Studies have shown that under the stimulation of particularly interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and other pro-inflammatory factors, MSCs can inhibit T cell proliferation and induce activated T cell apoptosis by producing indoleamine-2,3-dioxygenase 1 (IDO-1) [25]. Cytokines such as transforming growth factor-β (TGF-β) and hepatocyte growth factor (HGF) secreted by MSCs can down-regulate the expression of cyclin D2 and up-regulate the expression of p27kip1, causing the cell cycle of T cells to be arrested in the G1 phase. Thus inhibiting the proliferation of T cells [26]. In addition, cytokines secreted by MSCs can induce Treg cells by inhibiting pro-inflammatory T cells, resulting in a decrease in the expression of TNF-α and IL-12, and an increase in the expression of IL-10. When the concentration of IL-10 in the microenvironment reaches a certain level, soluble human leukocyte antigen g5 (HLA-G5) will be secreted by MSC to inhibit the activation of TH1 and TH17 in CD4+ T cells and induce the production of Treg cells [27]. Therefore, the cytokines secreted by MSCs can not only inhibit the activation of immature T cells, but also change the differentiation process of T cell subsets. By activating the Fas/FasL signaling pathway between mesenchymal stem cells and T cells, inflammatory T cell apoptosis can be induced [28]. In addition, the Jagged-1/Notch1 signaling pathway is also involved in the immune regulation of T cells by MSCs, that is, by increasing the ratio of Jagged-1/Notch1 to promote the differentiation of CD4+ cells and Treg cells [29]. Although MSCs have strong immunomodulatory capacities, there are two key issues that need to be resolved in the current MSC intravenous treatment research: (1) Cell heterogeneity, which is the main reason why it is difficult to predict the lasting effect of cell-based therapy [30]; (2) MSCs are rapidly phagocytic by monocytes after intravenous injection, and it is difficult to circulate to substantive organs through body fluids [31].
By comparing the immunomodulatory capabilities of P5 hucMSCs from five different donor sources, our group found that the five hucMSCs strains had heterogenous regulatory abilities on CD3+ cells, Th1/Th17 cell subsets and Treg cell subsets. Such problems may lead to instability in the efficacy of hucMSCs based therapy in clinical trials. It is worth noting that five hUC-MSC strains have homogeneous inhibitory effects on the secretion of TNF-α. Therefore, the TNF-α inhibition rate of more than 90% can be used as the criterion for judging the effectiveness of umbilical cord mesenchymal stem cells. This study provides a reference for the experimental data to quantify the immunomodulatory ability of hucMSCs. Xie et al. [32] found that three strain of huMSCs with promoting Treg cells, suppressing Th1 cell subpopulation and suppressing Th17 cell subpopulation were screened respectively and then applied to mouses fibrosis models. The results showed that compared with the other two strains of hucMSCs, the hucMSC with the specific immunomodulatory effect of Treg cell subsets significantly increased alanine aminotransferase (ALT) level and mRNA levels of Foxp3 in splenocytes. In addition, the hucMSC with the specific immunomodulatory effect of Th1 cell subsets significantly decreased the mRNA level of IFN-γin splenocytes. Based on the above experiments and our experimental data, by comparing the inhibition rate and promotion rate of immune cell regulation, hucMSCs with strong immunity or superiority in the treatment of specific diseases can be screened out before clinical application.
Exosomes are derivatives of MSCs, which are safer, more stable, and have stronger application potential. MSCs-derived exosomes can modulate immune function, which can induce macrophage polarization and immune suppression of effector T cells and induce the production of Tregs [33]. Studies have shown that miR-223 contained in exosomes derived from MSCs for skin wound healing can induce the polarization of macrophages by targeting pknox1 [34]. Chen et al. found that by co-cultivating peripheral blood mononuclear cells with exosomes derived from MSCs, exosomes can induce TH1 cells to transform into TH2 cells and reduce the potential of T cells to differentiate into TH17 cells, thereby increasing the content of Tregs for immunity adjust [35]. In addition, the study by Baharlooi et al. showed that MSC-derived exosomes could be used as an effective cell-free therapy to inhibit the proliferation of PBMC, and the inhibitory effect of hucMSCs-ex on PBMC is more effective than that of hucMSC [20]. However, whether the immune regulation of hucMSCs-ex must be stronger than that of HU-MSCs, there are different voices in the research field. Recent studies have suggested that exosomes have almost no effect on B cells [36]. This difference in research results may be caused by a variety of factors, including the source of MSCs, isolation methods, culture conditions, and/or the use of repeated freezing and thawing of exosomes. It is also possible that exosomes derived from MSC itself have no immunomodulatory function on B cells. Therefore, our experiment studied the differences in immunomodulatory capacity of hucMSCs and their derivatives to better improve the homogeneity and efficacy of the therapeutic effects of MSCs and their derivatives.
In this study, the immunomodulatory capacity of five strains of P5 hucMSCs from different donor sources and their exosomes were compared. The data showed that exosomes had no inhibitory effect on CD3+ cell proliferation, but increased apoptosis of CD4+ cell population, which was consistent with the results of Del Fattore et al [20]. In addition, exosomes derived from huc-MSC had no inhibitory effect on Th1 cell subsets, but increased the proportion of Th2 cell subsets. In this study, both MSCs and their derived exosomes showed significant inhibitory effect on Th17 cell subsets. Although the inhibitory rates of the hucMSCs-ex experimental groups were lower than that of the hucMSC experimental groups, the inhibitory effect of the hucMSCs-ex experimental groups was more homogeneous than that of the hucMSC experimental groups. In addition, hucMSC and their exosomes all had significant promotion effects on Treg cell subsets. Although the promotion rates of hucMSCs-ex experimental groups were lower than that of stem cells, the promotion effect of hucMSCs-ex experimental groups was more homogeneous than that of hucMSC experimental groups. It was also proved that the inhibition rate of TNF-α in hucMSC experimental groups was higher than that in hucMSCs-ex experimental groups. In this study, the immunomodulatory capacity of P5 hucMSCs-ex is weaker than that of P5 huc-MSC from five different donor sources, but hucMSCs-ex groups have better immunomodulatory homogeneity. It is a future research direction to improve the therapeutic targeting and immune regulation ability of exosomes through pharmacological compound pretreatment of MSCs, transgenic MSCs or miRNA-loaded exosomes and peptide-labeled exosomes, etc. [4]. The results show that the exosomes of mesenchymal stromal stem cells pretreated with LPS have a better ability to regulate macrophage balance and reduce inflammation than untreated MSC-derived exosomes by regulating the let-7b/TLR4 pathway [36]. Huang et al [37] showed that EV derived from transgenic MSC (BMP2 overexpression) can be effectively used as a biomimetic substitute for growth factors to enhance tissue-specific regeneration (bone regeneration) in vivo. In order to make better use of stem cell exosomes as immunomodulatory tools, while ensuring the safety and effectiveness of treatment, more relevant in vitro and in vivo studies are needed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 82271435)
Conflict of interest
None
References
- Dominici, M., et al., Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement.Cytotherapy, 2006. 8(4): p. 315-7.
- Wang, L., et al., Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A Prospective Phase I/II Study.Drug Des Devel Ther, 2019. 13: p. 4331-4340.
- Xiong, J., et al., Mesenchymal Stem Cell Exosomes as a New Strategy for the Treatment of Diabetes Complications.Front Endocrinol (Lausanne), 2021. 12: p. 646233.
- Wang, X., et al., The Application Potential and Advance of Mesenchymal Stem Cell-Derived Exosomes in Myocardial Infarction.Stem Cells Int, 2021. 2021: p. 5579904.
- Li, T., et al., Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy.Expert Opin Biol Ther, 2015. 15(9): p. 1293-306.
- Fischer, U.M., et al., Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect.Stem Cells Dev, 2009. 18(5): p. 683-92.
- Colombo, M., G. Raposo, and C. Théry, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles.Annu Rev Cell Dev Biol, 2014. 30: p. 255-89.
- Cui, G.H., et al., RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease.Immun Ageing, 2019. 16: p. 10.
- Mathivanan, S. and R.J. Simpson, ExoCarta: A compendium of exosomal proteins and RNA.Proteomics, 2009. 9(21): p. 4997-5000.
- Wahlgren, J., et al., Delivery of Small Interfering RNAs to Cells via Exosomes.Methods Mol Biol, 2016. 1364: p. 105-25.
- O’Loughlin, A.J., C.A. Woffindale, and M.J. Wood, Exosomes and the emerging field of exosome-based gene therapy.Curr Gene Ther, 2012. 12(4): p. 262-74.
- Sun, L., et al., Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell.Cytotherapy, 2016. 18(3): p. 413-22.
- Yaghoubi, Y., et al., Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment.Life Sci, 2019. 233: p. 116733.
- Hua, J., et al., Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow.Cell Biol Int, 2013.
- Salehinejad, P., et al., Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly.In Vitro Cell Dev Biol Anim, 2012. 48(2): p. 75-83.
- Van Pham, P., et al., Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications.Cell Tissue Bank, 2016. 17(2): p. 289-302.
- Rad, F., et al., Microvesicles preparation from mesenchymal stem cells.Med J Islam Repub Iran, 2016. 30: p. 398.
- Tan, K.L., et al., Benchtop Isolation and Characterisation of Small Extracellular Vesicles from Human Mesenchymal Stem Cells.Mol Biotechnol, 2021. 63(9): p. 780-791.
- Chen, W., et al., Immunomodulatory effects of mesenchymal stromal cells-derived exosome. 64(4): p. 831-840.
- Del Fattore, A., et al., Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes.Cell Transplant, 2015. 24(12): p. 2615-27.
- Liu, M., et al., [Study of immunomodulatory function of exosomes derived from human umbilical cord mesenchymal stem cells].Zhonghua Yi Xue Za Zhi, 2015. 95(32): p. 2630-3.
- Luz-Crawford, P., et al., Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells.Stem Cell Res Ther, 2013. 4(3): p. 65.
- Shafei, A.E., et al., Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction.J Gene Med, 2017. 19(12).
- Xie, M., et al., Immunoregulatory Effects of Stem Cell-Derived Extracellular Vesicles on Immune Cells.Front Immunol, 2020. 11: p. 13.
- Behm, C., et al., Cytokines Differently Define the Immunomodulation of Mesenchymal Stem Cells from the Periodontal Ligament. 9(5): p. 1222.
- Kyurkchiev, D., et al., Secretion of immunoregulatory cytokines by mesenchymal stem cells.World J Stem Cells, 2014. 6(5): p. 552-70.
- Selmani, Z., et al., Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. 26(1): p. 212-222.
- Akiyama, K., et al., Mesenchymal-Stem-Cell-Induced Immunoregulation Involves FAS-Ligand-/FAS-Mediated T Cell Apoptosis. 10(5): p. 544-555.
- Karamzadeh, R., M. Baghaban Eslaminejad, and A. Sharifi-Zarchi, Comparative In Vitro Evaluation of Human Dental Pulp and Follicle Stem Cell Commitment.Cell J, 2017. 18(4): p. 609-618.
- Jiang, W. and J.J.C.P. Xu, Immune modulation by mesenchymal stem cells.2020(5411).
- Wang, J.H., et al., Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity: A systematic review.World J Stem Cells, 2020. 12(8): p. 879-896.
- Xie, Y., et al., Individual heterogeneity screened umbilical cord-derived mesenchymal stromal cells with high Treg promotion demonstrate improved recovery of mouse liver fibrosis.Stem Cell Res Ther, 2021. 12(1): p. 359.
- He, X., et al., MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing.Stem Cells Int, 2019. 2019: p. 7132708.
- Baharlooi, H., et al., Umbilical cord mesenchymal stem cells as well as their released exosomes suppress proliferation of activated PBMCs in multiple sclerosis.Scand J Immunol, 2021. 93(6): p. e13013.
- Carreras-Planella, L., et al., Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. 10: p. 1288-.
- Ti, D., et al., LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b.J Transl Med, 2015. 13: p. 308.
- Huang, C.C., et al., Functionally engineered extracellular vesicles improve bone regeneration.Acta Biomater, 2020. 109: p. 182-194.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/