J Med Discov (2021); 6(1):jmd20068; DOI:10.24262/jmd.6.1.20068; Received September 05th, 2020, Revised October 30th, 2020, Accepted November 20th, 2020, Published January 11th, 2021.
Plant protease inhibitors and their antiviral activities – Potent therapeutics for SARS CoV-2
Shruti Gupta1, Shamsher Singh Kanwar1,*
1 Department of Biotechnology, Himachal Pradesh University, Summer Hill, Shimla-171 005, India .
* Correspondence:Shamsher Singh Kanwar,Department of Biotechnology, Himachal Pradesh University, Summer Hill, Shimla-171 005, India. Email: kanwarss2000@yahoo.com
Abstract
Protease inhibitors are highly active diverse family of poly(peptides) that are generally present in high concentrations in the storage tissues of the plants such as seeds and tubers. They play important roles in the regulation of proteases and the defence mechanism of plants against pathogens and display antimicrobial, antitumor and antiviral properties. Protease inhibitors have proved to be pharmacologically efficient tools in curing infections and systemic diseases via control of proteolysis. Recently, the outbreak of coronavirus (COVID-19) from Wuhan city of China has caused a global pandemic which has put the entire world on a standstill. Although the entire world has diverted all their efforts in finding an appropriate preventive and cure strategy, yet till date no success has been obtained. Since various viral diseases have been successfully cured by inhibition of viral proteases which are necessary for proteolytic processing of polyproteins, the inhibition of the proteases present on the surface of SARS-CoV-2 using protease inhibitors could prove to be fruitful in the treatment of this disease. This review gives a detail information of several natural protease inhibitors present in plants and their antiviral potential. The phytomolecules may be used for prophylaxis and effective therapeutics for the ongoing COVID-19 disease.
Keywords:Plant protease inhibitors; COVID-19; serpins; antiviral natural compounds; therapeutics
Key highlights
- Plants are natural sources of protease inhibitors (PIs).
- In plants, PIs are known to act in the defence mechanism against pathogens.
- Plant PIs have been known to possess antiviral activities against several pathogenic viruses such asHIV, Hepatitis C virus and human cytomegalovirus (HCMV).
- Plant PIs can inhibit the main protease (Mpro or 3CL) of SARS-CoV-2 essential for processing of the polyproteins of the virus into functional proteins.
- Plant PIs also act as inhibitory molecules against TMPRSS2, a transmembrane protein present on the host cell, required by the virus to enter into the host cell.
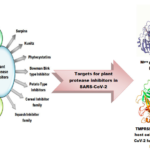
Graphical abstract
Introduction
Proteases (peptidases or proteinases) are one of the most important class of hydrolytic enzymes with discernible roles in several physiological and biochemical processes. Even though these enzymes are essential for maintenance and survival of their host as they are involved in signal transduction, protein-protein interaction, inflammatory response, protein catabolism, blood coagulation and digestion, their regulation is very crucial as they as they can be potentially harmful [1.2].
Protease inhibitors (PIs) are highly active compounds which are involved in important physiological reactions related to metabolism, cell physiology and regulation of proteolytic action. In a number of biological pursuits like blood clotting, apoptosis, hormone processing and inflammation, the PIs are now treated as very important signaling molecules [3]. They are widely distributed in plants and animals. In plants, PIs are present as small proteins in the storage tissues such as seeds and tubers in high concentrations and in other tissues they exist in low concentrations. Plant PIs act as storage proteins in the form of nitrogen sources, are also involved in modulation of enzymatic processes, regulation of apoptosis and defence mechanism against animals, insects and microorganisms [4]. Plant PIs possess a notable resistance to heat treatment and a high stability against alterations in ionic strength, pH, proteolysis as well as denaturing agents due of the high content of cysteine residues in disulfide bridges [5]. Several recent investigations report novel biologic activities for plant PIs such as antimicrobial activities, anticoagulant activities, antioxidant action as well as inhibition of tumor-cell growth; thus marking them potent molecules for inactivating proteases involved in several human diseases like arthritis, pancreatitis, thrombosis, emphysema, hypertension, cardiovascular morbidities, neurodegenerative diseases (such as Alzheimer’s disease) and muscular dystrophy. They have been employed in several fields of biotechnology and agriculture and control of the spread of several pathogens that cause life threatening diseases like cancer, AIDS, hepatitis, malaria and various others have proved to be prevented by using plant PIs in drug design [5]. In order to be used as therapeutics in humans, the PIs should be capable of inhibiting each of the major intestinal proteases, such as pancreatic trypsin, α-chymotrypsin, as well as elastase and must be nontoxic, too. PIs are being commercially used for deterrence of protease-induced perianal dermatitis and several nontoxic PIs have been isolated and purified from barley seeds, cabbage leaves and Streptomyces [6].
PIs are found in plants belonging to a variety of systematic groups especially those belonging to the Solanaceae family harbour several high levels of PIs. [6]. In plants, PIs were first discovered as chymotrypsin and trypsin inhibitors in tomatoes infected with Phytophthora infestans and were correlated to plant resistance to pathogens [7]. Later serine PIs of 20-24 kDa were found in potato tubers in response to infection with P. infestans and mechanical wounding [8,9].
Classification of plant protease inhibitors
On the basis of primary and tertiary structure, including the number and position of disulphide bonds and active sites, PIs can be classified in four groups according to the class of proteases they inhibit: serine protease, cysteine protease, metallocarboxy-protease or aspartic protease [10]. Based on structural and biochemical properties, plant PIs have also been classified as serpins and Bowman-Birk serine (BBIs), cysteine, potato type I and type II PIs, cereal trypsin/α-amylase, mustard trypsin, squash inhibitors, metallocarboxypeptidase and soybean trypsin (Kunitz) inhibitors (Table 1). On the basis of their amino acid similarities and the structures obtained, 48 identified plant PIs have been grouped into 26 related superfamilies (or clans). According to the MEROPS database, the inhibitors have 82 family members [2]. Different classes of plant PIs exhibit different mechanisms through which they interact with the target proteases. Some of the PIs utilize an irreversible inhibition of proteolytic activity (e.g. serpins) while most of them exhibit a canonical-competitive inhibition mode via ‘substrate-like’ binding to the catalytic domain of the targeted protease (e.g., BBIs and Kunitz inhibitors) or they make use of a non-catalytically competitive inhibition (e.g. cystatins or mustard-type PI) else they may act via a mixed mode, where the primary competitive binding to the active site is supported by a secondary binding event [e.g. metalloprotease inhibitors; 11].
Table 1: Plant protease inhibitors of different families.
| Family | Protease inhibitor | Plant source | Characteristic | Reference |
| Serpins | At-serpin 1 | Arabidopsis thaliana | Acts against metacaspase in vivo and plays role in plant immunity | [32] |
| OSZa-d | Oat (Avena sativa L.) grain |
OSZa and OSZb are efficient inhibitors of pancreatic elastase.
OSZb is an inhibitor of chymotrypsin whereas OSZc is a fast inhibitor of trypsin.
Together they display a broader activity against the digestive serine proteinases than the other serpins from rye, barley or wheat. |
[33] | |
|
CmPS-1 (Cucurbita maxima phloem serpin-1) |
Cucurbita maxima |
Possess anti-elastase activity. May impart resistance against bacteria, insects and phytoplasma. |
[18,34] | |
| WSZI | Triticum aestivum | Inhibits chymotrypsin and Cathepsin G | [11] | |
| HorvuZx (BSZx) | Hordeum vulgare | Inhibits trypsin, chymotrypsin, Factor Xa, thrombin, Factor VIIa, plasma kallikarein and leukocyte elastase. | [11] | |
| Kunitz | Kunitz trypsin inhibitor |
Artocarpus Integrifolia (Jackfruit) |
Inhibits elastase, trypsin and chymotrypsin.
However, it displays a very poor action on Streptomyces caespitosus and Aspergillus oryzae proteases |
[35] |
| Tamarind Kunitz inhibitor | Tamarindus indica | Inhibits trypsin and Factor Xa | [11] | |
|
SKTI-3
|
Glycine max | Inhibits plasmin, human Factor XIIa, plasmin kallikrein, trypsin, chymoreypsin | [11] | |
|
PdKI-2
|
Pithecelobium dumosum seed |
Inhibits trypsin as well as papain, a cysteine protease. Active against digestive enzyme of larvae from diverse orders and hence can be used as a potent insect antifeedant |
[36] | |
| Kunitz inhibitor CPTI | Cicer arietinum |
Show differential inhibitory activity against trypsin, chymotrypsin, H. armigera gut proteases and bacterial protease(s) |
[37] | |
|
PFTI
|
Plathymenia foliolosa |
Inhibits bovine trypsin and bovine chymotrypsin.
Exhibits significant inhibitory activity against on larval midgut proteases of A. kuehniella and D. saccharalis |
[38]
|
|
| PCP1 6.6 and PCPI 8.3 | Solanum tuberosum |
Possesses inhibitory action against cathepsin B, H and L.
Also inhibits dipeptidyl peptidase I and Clostripain. |
[11] | |
| Bowman birk type inhibitors (BBI) |
Soybean BBI (Isotype 2-II; ̴8 kDa)
|
G. max | Inhibits trypsin, chymotrypsin, mast cell chymase, cathepsin G, matriptase, leukocyte elastase, duadenase | [11] |
| BTCI | Vigna unguiculata (black eyed pea) |
Trypsin/chymotrypsin inhibitor.
Moderately active against the digestive chymotrypsin of adult insects |
[39] | |
| AsPIs | Acacia Senegal seeds |
Highly active against serine proteases. Possess remarkable inhibitory activity towards total gut proteolytic enzymes followed by trypsin and chymotrypsin and retards the growth and development of H. armigera |
[40] | |
| BI-I (seven isotypes I-VII) | Ananas comosus | Possesses inhibitory activity against trypsin, papain, bormelain, cathepsin L and chymotrypsin | [11] | |
| Phytocystatin | Oryzacystatin I and II |
Oryza sativa
|
Inhibits cathepsin B, Hand L and Legumain | [41,42] |
| SQAPI | Cucurbita maxima | Inhibits pepsin proteases | [43] | |
| Corn cystatin-I | Zea mays | Inhibits Cathepsin H and L | [44] | |
| Potato inhibitor family | CI-1 | H. vulgare | Inhibitor of trypsin, chymotrypsin, subtilin and neutrophil elastase | [45] |
| PSI- 1.1 | Capsicum annuum | Trypsin and chymotrypsin inhibitor | [11] | |
| TI-II | Solanum lycopersicum | Inhibitor of trypsin, chymotrypsin and subtilisin | [46] | |
| PI-2 | S. tuberosum | Trypsin and chymotrypsin inhibitor | [11] | |
| Cereal inhibitor and squash inhibitor family |
Corn Hageman factor inhibitor
|
Z. mays | Inhibitors of serine proteases and α amylase | [47] |
| RATI | Eleusine coracana (ragi) | Inhibitors of serine proteases and α amylase | [48] | |
| BTI-CMe | H. vulgare (barley) |
Trypsin inhibitor;
Exhibits in vitro inhibition of trypsin-like proteases of the gut extracts of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). |
[18] |
Serine PIs or serpins constitute a major class of plant PIs, which have been classified into more than 20 families. Serpins are mainly found in plants belonging to the Solanaceae, Fabaceae, Euphorbiaceae, Poaceae, and Cucurbitaceae families [12.13] with most of these being isolated from barley grain, wheat grain, rye, wild oats, pumpkin and A. thaliana [4]. In plants they are responsible for controlling protein synthesis and turnover besides physiological functions such as fertilization, growth & development, digestion, cell signaling or migration, immune defense, wound healing and disease progression. They play crucial role(s) in the pathogenesis and/or host tissue penetration of a number of diseases, such as cardiopulmonary disease and emphysema [14]. Serpins display a distinctive mechanism of irreversible inhibition termed as “suicide substrate” mechanism rather than the standard reversible inhibition mechanism followed by other PIs. They are metastable proteins with a molecular weight usually higher than 40 kDa [15].
A report demonstrated that in serine PIs, the ‘reactive sites’ are mutating faster than amino acids in rest of the proteins, implying that their roles in defense against microorganisms (and insects) may exert a strong selection pressure on these proteins to conserve the reactive sites and that this selection may be related to plant defense [16]. Serine PIs also called serpins inhibit both serine and cysteine proteases [17]. Although several serpins with inhibitory activity against caspases and papain like cysteine proteases have been reported but they predominantly act against trypsin like serine proteinases [18].
Another important class of PIs is the inhibitors of the cysteine proteases (cystatins or phytocustatins) which range in molecular weight from 10 kDa to 23 kDa. They inhibit cysteine proteases in a non-catalytically competent manner (i.e. although they do not bind to proteases in a strictly substrate-like manner but they still block access to the catalytic site; [11]. Cystatins regulate endogenous and heterologous cysteine proteases in a variety of physiological processes such as abiotic stress tolerance, protection against insects and nematodes via inhibition of digestive enzymes in their gut, regulation of peptidase activity during apoptosis, protection of cytosolic metabolism from intracellular peptidases released by incidental rupturing of protein bodies. They have been isolated and characterized from a number of vegetables and crop plants such as cabbage, apple, papaya, avocado, carrot, cowpea, ambrosia, castano, seeds of wheat, maize, sunflower, soybean, sugarcane, rice etc [5].
The Kunitz and BBI have been observed in the leguminous family and they generally range in size from 18-24 and 5-16 kDa, respectively. Both of them function via competitive inhibition of protease using the standard mechanism of substrate like binding to the catalytic site of the protease. Kuntiz inhibitors are known to function in the regulation of physiological homeostasis and in inhibition of pathogenic proteases while the expression of BBIs in plants is strongly induced by pathogenic invasion [11]. Other than this, a few aspartate and metalloprotease inhibitors have been reported which are isolated from potato tubers, sunflower flowers, barley and thistle (Cynara cardunculus). Metalloprotease are highly compact and stable proteins in nature because of the high content of disulphide bonds in them [5].
Antiviral potential of plant PIs
According to various reports, serine PIs in plants provide protection against various pests and pathogens. In most of the pathogenic organisms like bacteria, fungi, viruses, insects and vertebrates, proteases comprise around 1-5% of the genome among which majority of the functions are performed by serine proteases [14]. The NS3 protein of Hepatitis C virus (HCV) is a chymotrypsin like protein which contains a serine protease domain that is responsible for processing of the HCV polyprotein. The Human cytomegalovirus (HCMV) contains a Ser-His-His catalytic triad and therefore is a serine protease which is essential for capsid formation during viral replication [19,20]. Therefore, serpins can be effectively utilized to attenuate such serine proteases thereby providing protection against a wide variety of pathogens. Novel antiviral strategies include targeting either host or viral accessory protein to ultimately block viral replication or inhibit cellular proteins necessary for the virus life cycle. Proteolytic cleavage of the precursor hemagglutinin (HA0) into HA1 and HA2 subunits by host proteases is essential for fusion of HA with the endosomal membrane and thus represents an essential step for viral infection [14]. The trypsin PIs from the leaf extract of Capsicum baccatum var. pendulum inoculated with Pepper yellow mosaic virus (PepYMV) significantly reduced the yellow mosaic viral infection [21]. The Cucumis metuliferus serine PIs (CmSPI) gene when overexpressed and silenced in Nicotiana benthamiana and Cucumis metuliferus displayed potyvirus resistance and synchronous development of potato ring spot viral symptoms, respectively [14]. The sunflower trypsin inhibitor (TI) from Helianthus annuus is the smallest known Bowman birk type inhibitors (BBI) which has been explored as a model peptide for drug design [22-24]. Various plant PIs displaying antiviral activity have been previously reported (Table 2).
Table 2: Prominent plant protease inhibitors with antiviral activity.
| Protease inhibitor | Plant source | Antiviral activity | Reference |
| Capsicum baccatum trypsin protease inhibitor | Capsicum baccatum var. Pendulum | Reduction in the yellow mosaic virus infection of Capsicum baccatum | [21] |
| BSKTI (Kunitz trypsin protease inhibitor) | G. max cv.Dull Black seeds | Anti HIV-1 reverse transcriptase activity | [27] |
| BvvTI (Kunitz trypsin protease inhibitor) | B. variegate seeds | Anti HIV-1 reverse transcriptase activity as well as antitumor activity against human nasopharyngeal cells | [25] |
| KBTI (Kunitz trypsin protease inhibitor) | G. max seeds | Anti HIV-1 reverse transcriptase activity as well as antitumor activity against human nasopharyngeal cells, breast cancer cells and hepatoma cells | [26] |
| Coumarin derivatives (aspartyl protease inhibitor) | Fruits such as (bilberry, cloudberry), green tea, chicory, soy, higher plants such as Rutaceae and Umbelliferone, stem bark of Calophyllum dispar (Clusiaceae) | Act against aspartyl proteases of retroviruses such as HIV. Potential therapeutic for malaria, Q fever, mycoplasmosis and, nucleoplasmosis | [28,29] |
| CmSPI (Cucumis metuliferus serine protease inhibitor) | Cucumis metuliferus | Overexpression of the gene provides resistance to potyvirus in Nicotiana benthamiana; Silencing of the CmSPI gene in Cucumis metuliferus results in development of potato ring spot viral symptoms | [14] |
| Novel trypsin-chymotrypsin inhibitor | Vicia faba (bakla) seeds | Anti HIV-1 reverse transcriptase activity as well as antifungal activity against Mycosphaerella arachidicola and Physalospora piricola. | [30] |
| Chymotrypsin inhibitor | Acacia confusa seeds | Anti HIV-1 reverse transcriptase activity | [49] |
The Kunitz trypsin inhibitors isolated from B. variegate and G. max seeds termed BvvTI and KBTI, respectively, display significant activity against the HIV-1 reverse transcriptase. They also possessed anti-tumor activity against the human nasopharyngeal cancer cells, human breast cancer cells and hepatoma cells [25,26]. Another Kunitz trypsin inhibitor, BSKT1 isolated from G. max cv. Dull black seeds also possessed anti HIV-1 reverse transcriptase activity [27]. According to the invention of Domagala et al., the derivatives of coumarin which is found in fruits (bilberry and cloudberry), green tea, chicory, soy, higher plants such as Rutaceae and Umbelliferone as well as the stem bark of Calophyllum dispar (Clusiaceae) are inhibitors of aspartyl proteases, especially the aspartyl proteases of retroviruses such as HIV and hence can be expected to be used as an antiviral agent in the treatment of retroviral infections. They also have been found to be potential therapeutics for treatment of malaria, mycoplasmosis, Q fever and mononucleosis [28,29]. Ye and Ng in 2002 isolated a novel trypsin chymotrypsin inhibitor from Vicia faba (commonly known as bakla in India) seeds which displayed anti HIV-1 reverse transcriptase activity as well as antifungal activity against Mycosphaerella arachidicola and Physalospora piricola [30]. A novel, fairly stable Kunitz trypsin inhibitor of serpin family was isolated from Allium sativum (garlic) by Shamsi and colleagues which could act as a potential non toxic therapeutic against a number of viral diseases [31].
The COVID-19 pandemic
In December 2019, the city of Wuhan, the capital of Hubei province in China, reported the outbreak of a pulmonary disease caused by a novel strain of coronavirus and since then the virus has spread globally [50]. The spread of 2019-nCoV, now officially known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still progressing world over despite of the severe containment measures being taken [51]. The virus consists of an RNA genome which is 82% identical to the SARS coronavirus (SARS-CoV) and both viruses belong to clade b of the genus Betacoronavirus and hence it has been named as SARS-CoV-2 and the disease caused by SARS-CoV-2 is called COVID-19 [52, 53]. Although less is known about the origin of the virus but on the basis of the sequence of the viral genome and the evolutionary analysis, bats have been suspected as their natural hosts and it has been supposed that in humans SARS-CoV-2 might have been transmitted from bats via some unknown intermediate host [54]. Within humans, the disease is transmitted by inhalation or contact with infected droplets released by an infected person and the incubation period ranges from 2 to 14 d. The symptoms usually consist of fever, cough, sore throat, breathlessness, fatigue, malaise etc. Although the disease is mild in most people; but in some (usually the elderly and those with comorbidities), it may advance to pneumonia, acute respiratory distress syndrome (ARDS) and multi organ dysfunction. Many people are asymptomatic. The case fatality rate is estimated to range from 2 to 3%. It was listed as a potential global health emergency by WHO due to high mortality, high basic reproduction number and lack of clinically approved drugs and vaccines for COVID-19. India too has reported more than 92,700,00 of coronavirus cases along with 1,35,000 deaths all over the country till Nov. 26, 2020.
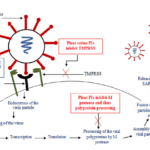
The replication cycle of the SARS-CoV-2 virus has been illustrated (Figure 1) to focus on therapeutics for efficient neutralization of virus or inhibition of some intervening virus adsorption or replication step(s). For entry into the host cell, the viral S protein binds to the host cellular receptor angiotensin converting enzyme 2 (ACE2). The binding requires the host cell surface associated trans-membrane protease serine 2 (TMPRSS) for cleavage of the trimeric S protein [54]. After binding of the S protein and ACE2, there occurs a conformational change in the S protein which facilitates the fusion of the viral envelope with the host cell’s membrane through endosomal pathway. After entry into the host cell, the virus un-coats itself and releases its RNA, which is replicated and translates into viral replicase polyproteins. The polyproteins are then processed into functional proteins by the main protease of SARS-CoV-2, Mpro also called as 3CL protease. The viral proteins and the genomic RNA subsequently assemble into virions in the endoplasmic reticulum and Golgi and subsequently released out of the cell [55].
Figure 1: Schematic representation of replication cycle of the SARS-CoV-2 and the potent inhibitory effects of plant PIs on its replication in human cells.
Potent inhibitory effects of plant PIs on SARS CoV-19
Most of the nation’s world-wide have been diverting their best efforts for the implementation of appropriate preventive and control strategies to deal with SARS CoV-19. Neither vaccines nor direct-acting antiviral drugs are available for the treatment of human and animal coronavirus infections [56]. The inhibition of viral proteases necessary for proteolytic processing of polyproteins has been a successful strategy in the pharmacological treatment of HIV and HCV, respectively, proving the potential of PIs for the treatment of viral infections. Similarly, the main protease of SARS-CoV-2, Mpro or 3CL is thought to be essential for viral replication and therefore, is regarded as promising target for plant PIs and antiviral pharmacotherapy [Figure 1; 57]. Inhibiting the activity of this enzyme would block viral replication in the infected host cells. Since no human proteases with similar cleavage specificity are known, inhibitors are unlikely to be toxic. Approved PIs including disulfiram, lopinavir and ritonavir have been reported to be active against SARS and MERS. Disulfiram, an approved drug to treat alcohol dependence, has been reported to inhibit the papain-like protease of MERS and SARS in cell cultures in vitro, but clinical evidence is lacking. According to the observation of Baden and colleagues, lopinavir–ritonavir combination did not seem to be highly effective in patients with COVID-19 [58] and adverse gastrointestinal effects were seen in approximately 13% of the patients [59]. Since better effective therapies for COVID-19 is the demand of the moment and plant PIs may prove to be potential therapeutic agents by inhibiting this main protease of the virus.
As described before, TMPRSS plays a major role in 2019-nCoV infection as it is the main protease which allows the fusion of the virus particles with human cells. Hence, because TMPRSS is required by the COVID-19 virus to enter into the human cells, the inhibition of this protease by non toxic plant serine PIs may prove to be potential treatment options in 2019-nCoV infection [Figure 1; 60].
Conclusion
The review suggests that PIs are widely distributed in several plants where they play important role(s) in providing defense against pathogenic diseases. The plant PIs have been classified into different families on the basis of their structural similarity and protease inhibited. Because of their non toxic nature and fairly good stability, they have been employed in several biotechnological and pharmaceutical applications. The PIs are effective tools in inhibiting proteases associated with a number of diseases. They are also highly efficient in inhibiting viral proteases, they can be employed as a potential therapeutic in the treatment of the ongoing COVID-19 pandemic which has been declared by the WHO as a global emergency. Further docking and in vivo studies are required for finding the possible use of these plant PIs in the treatment of COVID-19.
Acknowledgments
The authors are grateful to the Department of Biotechnology, Himachal Pradesh University, Shimla, for the providing various resources. We are also thankful to the Department of Biotechnology, Government of India, for providing the necessary support to the authors. Further, the authors received no funding sources for this work.
Conflict of interest
Both the authors declare that they have no conflict of interest among themselves at their place of work or with the institution.
References
- Clemente M, Corigliano MG, Pariani SA, Sánchez-López EF, Sander VA, Ramos-Duarte VA. Plant serine proteaseinhibitors: biotechnology application in agriculture and molecular farming. Int J Mol Sci. 2019;20-1345.
- Fan Y, Yang W, Yan Q, Chen C, Li J. Genome-wide identification and expression analysis of the protease inhibitor gene families in tomato. Genes.2020;11-1. doi:10.3390.
- Srikanth S, Chen Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front Pharmacol.2016;7:470.
- Volpicella M, Leoni C, Costanza A, Leo FD, Gallerani R, Ceci LR. Cystatins, serpins and other families of protease inhibitors in plants.. Curr Prot Pept Sci 2011;12:386-398.
- Cotabarren J, Lufrano D, Parisi MG, Obreg´on WD. Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Plant Sci.2019;S0168-9452(19)31571-7.
- Kim JY, Park SC, Hwang I, Cheong H, Nah JW, Hahm KS et al. Protease inhibitors from plants with antimicrobial activity. Int J Mol Sci.2009;10:2860-2872.
- Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, van den Elzen PJ, Cornelissen BJ. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991;3:619-628.
- Valueva TA, Revina TA, Kladnitskaya GV, Mosolov VV. Kunitz-type proteinase inhibitors from intact and Phytophthora infected potato tuber. FEBS Lett. 1998;426: 131-134.
- Valueva TA, Revina TA, Gvozdeva EL, Gerasimova NG, Ozeretskovskaya OL (2003). Role of proteinase inhibitors in potato protection. Bioorg Khim. 2003;29:499-504.
- Laskowski M Jr., Kato I (1980). Protein inhibitors of proteinases. Annu Rev Biochem.1980;49:593-626.
- Hellinger R, Gruber CW. Peptide-based protease inhibitors from plants. Drug Discov Today; 2019;24:1877–1889.
- Haq SK, Atif SM, Khan RH. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection. Arch Biochem Biophys.2004;431:145-159.
- Fan SG, Wu GJ. Characteristics of plant proteinase inhibitors and their applications in combating phytophagous insects, Bot Bull Acad Sin.2005;46:273–292.
- Mishra UN, Reddy MV, Prasad DT. Plant serine protease inhibitor (SPI): A potent player with bactericidal, fungicidal, nematicidal and antiviral properties. Int J Chem Stud.2020;8:2985-2993.
- Patston PA, Gettins PG. Significance of secondary structure predictions on the reactive center loop region of serpins: a model for the folding of serpins into a metastable state. FEBS Lett. 1996;383;87-92.
- Ryan CA. Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annu Rev Phytopathol. 1990;28:425-49.
- Cohen M, Davydov O, Fluhr R. Plant serpin protease inhibitors: specificity and duality of function. J Exp Bot.2019;doi:10.1093/jxb/ery460.
- Jamal F, Pandey PK, Singh D, Khan MK. Serine protease inhibitors in plants: nature’s arsenal crafted for insect predators. Phytochem Rev. 2013;12:1–34.
- Bianchi E, Pessi A. Inhibiting viral proteases: Challenges and opportunities. Sci.2002;66:101-114.
- Fischmann TO, Weber PC. Peptidic inhibitors of the hepatitis C virus serine protease within non- structural protein 3. Curr Pharm Des. 2002;8:2533-2540.
- Moulin MM, Rodrigues R, Ribeiro SFF, Goncalves LSA, Bento CS, Sudre CP. Trypsin inhibitors from Capsicum baccatum pendulum leaves involved in Pepper yellow mosaic virus resistance. Genet Mol Res.2014;13:9229-9243.
- Craik DJ. Circling the enemy: cyclic proteins in plant defence. Trends Plant Sci.2009;14:328–335.
- Craik DJ, Fairlie DP, Liras S, Price D.The future of peptide-based drugs. Chem Biol Drug Des. 2013;81:136–147.
- Elliott AG, Delay C, Liu H, Phua Z, Rosengren KJ, Benfield AH et al. Evolutionary origins of a bioactive peptide buried within Plant Cell. 2014;26:981–995.
- Fang EF, Wong JH, Bah CSF, Lin P, Tsao SW, Ng TB. Bauhinia variegata var. variegata trypsin inhibitor: From isolation to potential medicinal applications. Biochem Biophys Res Commun. 2010;396:806–811.
- Fang EF, Wong JH, Ng TB. Thermostable Kunitz trypsin inhibitor with cytokine inducing, antitumor and HIV-1 reverse transcriptase inhibitory activities from Korean large black soybeans. J Biosci Bioeng.2010;109:211–217.
- Lin P, Ng TB. A stable trypsin inhibitor from Chinese dull black soybeans with potentially exploitable activities. Process Biochem. 2008;43:992–998.
- Domagala JM, Hagen SE, Lunney E, Arbor A, Tait BD. Coumarin derivatives as protease nhibitors and antiviral agents. US Patent. 1996;5,510,375.
- Jain PK, Joshi H (2012). Coumarin: Chemical and Pharmacological Profile. J Appl Pharm Sci. 2012;2:236-240.
- Ye XY, Ng TB. A new peptidic protease inhibitor from Vicia fabaseeds exhibits antifungal, HIV-1 reverse transcriptase inhibiting and mitogenic activities. J Pept Sci. 2002;8:656–662.
- Shamsi TN, Parveen R, Amir M, Baig MA, Qureshi MI, Ali S. Allium sativumprotease inhibitor: A Novel Kunitz trypsin inhibitor from garlic is a new comrade of the serpin family. PLoS one. 2016;11:e0165572.
- Vercammen D, Belenghi B, van de Cotte B. Serpin1 of Arabidopsis thalianais a suicide inhibitor for metacaspase 9. J Mol Biol. 2006;364: 625–636.
- Hejgaard J, Hauge S. Serpins of oat (Avena sativa) grain with distinct reactive centres and inhibitory specificity. Physiol Plant. 2002;116:155–163.
- Yoo BC, Aoki K, Xiang Yu. Characterization of Cucurbita maximaphloem serpin-1 (CmPS-1) a developmentally regulated elastase inhibitor. J Biol Chem. 2000;275:35122–35128
- Bhat AV, Pattabiraman PN. Protease inhibitors from jackfruit seeds. J Biosci.1989;14:351–365.
- Oliveira AS, Migliolo L, Aquino RO. Purification and characterization of a trypsin–papain inhibitor from Pithecelobium dumosumseeds and it’s in vitro effects towards digestive enzymes from insect pests. Plant Physiol Biochem. 2007;45:858–865.
- Harsulkar AM, Giri AP, Patankar AG, GuptaVS, Sainani MN, Ranjekar PK et al. Successive use of non-host plant protease inhibitors required for effective inhibition of Helicoverpa armigera gut proteases and larval growth. Plant Physiol. 1999;121:497–506.
- Silveira RV, Silva GS, Freire MGM. Purification and characterization of a trypsin inhibitor from Plathymenia foliolosa J Agric Food Chem.2008;56:11348–11355.
- Franco OL, Dos Santos RC, Batista JAN (2003). Effects of black-eyed pea trypsin/chymotrypsin inhibitor on proteolytic activity and on development of Anthonomus grandis. 2003;63: 343–349.
- Babu SR, Subrahmanyam B. Bio-potency of serine proteinase inhibitors from Acacia senegal seeds on digestive proteinases, larval growth and development of Helicoverpa armigera (Hu¨bner). Pesticide Biochem Physiol. 2010;98:349–358.
- Nagata K, Norio K, Keiko A, Soichi A and Masaru T. Three-dimensional solution structure of oryzacystatin-I, a cysteine proteinase inhibitor of the rice, Oryza sativa L. japonica. Biochem. 2000;39:14753–14760.
- Valadares NF,Dellamano M, Soares-Costa A, Henrique-Silva F, Garratt RC. Molecular determinants of improved cathepsin B inhibition by new cystatins obtained by DNA shuffling. BMC Struct Biol. 2010;10:30.
- Headey SJ, MacAskill UK, Wright MA, Claridge JK, Edwards PJB, Farley PC et al. Solution structure of the squash aspartic acid proteinase inhibitor (SQAPI) and mutational analysis of pepsin inhibition. J Biol Chem.2010;285:27019–27025.
- Abe M, Abe K, Iwabuchi K, Domoto C, Arai S. Corn cystatin I expressed in Escherichia coli: investigation of its inhibitory profile and occurrence in corn kernels. J Biochem. 1994;116:488–492.
- Polya GM. Protein and non-protein protease inhibitors from plants. Stud Nat Prod Chem. 2003;29:567–641.
- Barrette-Ng IH, NgKKS, Cherney MM‡, Pearce G, Ghani U, Ryan CA et al. (2003). Unbound form of tomato inhibitor-II reveals inter-domain flexibility and conformational variability in the reactive site loops. J Biol Chem. 2003;278:31391–31400.
- Mahoney WC, Hermodson MA, Jones B, Powers DD, Corfman RS, Reeck GR. Aminoacid sequence and secondary structural analysis of the corn inhibitor of trypsin and activated Hageman Factor. J Biol Chem. 1984;259: 8412–8416.
- Shivraj B, Pattabiraman TN. Natural plant enzyme inhibitors.Characterization of an unusual alpha-amylase/trypsin inhibitor from ragi (Eleusine coracana Geartn). Biochem J. 1981;193:29–36.
- Lam SK, Ng TB. A dimeric high-molecular-weight chymotrypsin inhibitor with antitumor and HIV-1 reverse transcriptase inhibitory activities from seeds of Acacia confusa. Phytomed Int J Phytother Phytopharmacol.2010;17:621–625.
- Wang Q, Zhao Y, Chen X, Hong A. Virtual screening of approved clinic drugs with main protease (3CLpro)reveals potential inhibitory effects on SARS-CoV-2. 2020;2020030144.
- Devaux CA, Rolain JM, Colson P, Raoult D (2020). New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents.2020;doi: https://doi.org/10.1016/j.ijantimicag.2020.105938.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nat. 2020;579:270–273.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG et al. A new coronavirus associated with human respiratory disease in China. Nat. 2020;579:265–269.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Med Res.2020;7:11.
- Shereen MA,Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccines Immunother. 2020;DOI: 1080/21645515.2020.1735227.
- Fischer A, Sellner M, Neranjan S, Lill MA, Smiesko M. Inhibitors for novel coronavirus protease identifed by virtual screening of 687 million compounds. Chem Rxiv Preprint. 2020;https://doi.org/10.26434/chemrxiv.11923239.v1
- Baden LR, Rubin EJ. Covid-19 — The search for effective therapy.N Engl J Med. 2019;DOI: 10.1056/NEJMe2005477
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G et al. A Trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;DOI: 10.1056/NEJMoa2001282.
- Meng T, Cao H, Zhang H, Kang Z, Xu D, Gong H et al. The transmembrane serine protease inhibitors are potential antiviral drugs for 2019-nCoV targeting the insertion sequence-induced viral infectivity. BioRxiv Preprint. 2020;doi: https://doi.org/10.1101/2020.02.08.926006.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/