J Med Discov (2019); 5(1):jmd19024; DOI:10.24262/jmd.5.1.19024; Received July 17th, 2019, Revised December 10th, 2019, Accepted Feb 10th, 2020, Published March 12th, 2020.
BVAN08 increases radiosensitivity in HepG2 cells through inhibition of DNA damage repair
Bo Zhang1,2, Lantao Liu3, Sai Hu4, Zi-JianYu5, Hua Guan2, Lijing Geng1, Ping-Kun Zhou2*
1College of Food Science and Engineering, Jinzhou Medical University, Liaoning Province, Jinzhou. 121000, China
2Department of Radiation Toxicology and Oncology, Beijing Key Laboratory for Radiobiology (BKLRB), Beijing Institute of Radiation Medicine, Beijing 100850, China
3Beijing Institute for Occupational Disease Prevention and Treatment, 50 Yikesong road, Xiangshan, Beijing, 100093, P.R. China
4Institute for Environmental Medicine and Radiation Health, the College of Public Health, University of South China, Hengyang, Hunan Province 421001, P.R. China
5Department of Hepatobiliary Surgery, the First Affiliated Hospital, University of South China, Hengyang, Hunan Province 421001, P.R. China;
* Correspondence: Ping-Kun Zhou, Department of Radiation Toxicology and Oncology, Beijing Key Laboratory for Radiobiology (BKLRB), Beijing Institute of Radiation Medicine, Beijing 100850, China. Tel: +86 1066931217. Fax: +86 1068183899. E-mail addresses: zhoupk@nic.bmi.ac.cn, zhoupkek@yahoo.com.cn.
Abstract
Liver cancer is one of the most serious cancers threatening human health. Not only because it is hard to recognize in the early stage, but also has the characteristics of easy metastasis and greatly affects the curative effect due to chemoresistance. By studying the effect of vanillin derivative BVAN08 (6-bromine-5-hydroxy-4-methoxybenzaldehyde) on DNA damage repair, we found BVAN08 inhibit DNA double-strand break injury repair. Moreover, BVAN08 inhibits the expression of DNA-PKcs which could enhance the radiation sensitivity by activating ATM and Chk2 signaling pathways. This observation provides a further theoretical basis for developing BVAN08 as a radiosensitizer.
Keywords: DNA damage, Radiosensitivity, DNA-PKcs
Introduction
DNA double-strand breaks are important DNA damage types caused by ionizing radiation which are closely related to cell death, gene mutation and malignant transformation of cells. One of the earliest reactions after double strand breaks is the phosphorylation of the C-terminal serine residue of histone H2AX which located near the breakpoint to form γ-H2AX. Phosphorylated γ-H2AX rapidly transduces DNA-damaging signals that lead to the activation of downstream molecular phosphorylation and triggering a series of biological cascade reactions and cytological reactions. γ-H2AX is the most important DNA damage sensor by far [1].
Ataxia telangiectasia mutated (ATM) is a member of the family of phosphatidylinositol 3-kinase kinases and plays a crucial role in triggering signaling pathways in mammalian cells and in response to ionizing radiation and other factors-induced DSB. A number of cell cycle checkpoint proteins that act as ATM kinase substrates have been identified, including checkpoint kinase 2 (Chk2), γ-H2AX and p53. In normal cultured non-irradiated cells, ATM kinase is present as an inactive dimer or advanced multimers. Ionizing radiation has also been found to induce multiple ATM phosphorylation sites, including Ser367, Ser1883, Ser1884, Ser1893, Thr1884, Thr1885, and phosphorylation of Ser367 and Ser1893 is also required for ATM activation [2]. After DSB occurs, Thr-1981 phosphorylates or separates into active monomer, which in turn activates its substrate. For example, at Thr-68 site of Chk2, activated Chk2 further phosphorylates Cdc25A and p53 which result in cell cycle progression stagnation [3].
Polo-like kinase (Plk1) is a highly conserved serine / threonine protein kinase and is an important regulator of eukaryotic cell mitosis [4-5]. In the process of G2-M transition, Plk1 plays a very important role in promoting the cell cycle from G2 to mitosis phosphorylates. Phosphorylation of Thr210 and activates its kinase activity and phosphorylates various substrates such as Cdc25 and Cyclin B. BVAN08 is a derivative of the natural flavoring agent vanillin which has previously been demonstrated to have antiproliferative effects on various cancer cell lines [6-8]. In this study, we investigated the changes of DNA-PKcs and cell cycle checkpoint proteins in DNA damage repair pathway through the combined action of BVAN08 drug and radiation. Meanwhile, the mechanism of BVAN08 in radiation sensitization was also investigated.
Materials and methods
Chemicals and Cell culture
HepG2 human hepatocellular carcinoma cells were preserved in four chambers of the Second Military Medical Academy, cultured in DMEM containing 10% fetal bovine serum and incubated at 37 ° C in a 5% CO2 incubator.
Reagents and materials
Vanillin derivative BVAN08 (6-bromine-5-hydroxy- 4-methoxybenzaldehyde) was provided by Dr Lin Wang (Beijing Institute of Radiation Medicine). The β-actin antibody was purchased from Santa Cruz (sc1616), the DNA-PKcs antibody was purchased from Santa Cruz (H-163), the γ-H2AX antibody was purchased from Upstar (05-636) and the Plk1 antibody was purchased from Invitrogen 37-7000), phosphorylated T20-Plk1 was purchased from ABCAM (mouse polyclonal, ab39068), phospho-T68-Chk2 antibody was purchased from CST (# 2661), Chk2 antibody was purchased from CST (# 2662). The peroxidase-labeled goat anti-mouse and goat anti-rabbit secondary antibody was purchased from Beijing Zhongsheng Co. (ZB2305, ZB2301).
DNA double-strand breaks detected by neutral single cell gel electrophoresis
HepG2 cells were seeded on 60 mm dishes treated with 40 μM BVAN08. Cells were irradiated with 4 Gy 60Co γ rays after adherent. Cells were harvested at different time points 1, 2, 4 and 8 h after irradiation. Cells were collected and mixed with low melting point (LMP) agarose at 37 °C which was placed on the top of the previous layer of 0.5% normal melting point (NMP) agarose on the slide. Covered with a coverslip and returned to 4 °C until solid. The coverslip was gently removed followed by adding some NMP agarose onto the slide. Repeat this step. The slide was placed in neutral lysis buffer (for DNA double-strand breaks detection), and subjected to electrophoresis. Thereafter, the slides were gently washed with neutralization buffer and stained with ethidium bromide. Slides were visualized and analyzed under a fluorescence microscope. DNA damage was expressed as tail moment combining comet tail length and the proportion of DNA migrating into the tail.
Protein expression was detected by western blot
Each well was loading 40 μg total proteins. 75V constant flow was used to transfer protein to NC membrane for 1.5 h after SDS-PAGE gel electrophoresis. Blocking solution (TBST with 5% nonfat dry milk) was blocked for 1 h at room temperature and washed with TBST at room temperature for 5 min for 3 times. The expression of cell cycle related proteins in each cell was detected by ECL. β-actin was used as internal control protein.
Results
BVAN08 inhibits radiation-induced DNA double-strand break repair
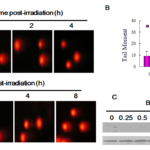
Tail movement experiment was used to investigate the effect of BVAN08 on radiation induced DNA double-strand break. The results showed that 4 Gy 60Co γ-ray irradiation has significant tailing in HepG2 cells after 1 h radiation which indicating DNA double-strand breaks was induced. Moreover, DSB had repaired at 4 hrs post-IR without other perturbation. However, pretreatment with 40 mM BVAN08 for 12 h, tail movement was detected even after 8 h of irradiation, indicating that DNA damage was occurred post irradiation and could not be repaired until 8 h (Figure 1A). Tail torque statistical analysis showed that BVAN08 not only induced DNA double-strand break injury but also inhibited irradiation-induced DNA double-strand break repair (Fig. 1B). Meanwhile, pretreatment of BVAN08 followed by radiation increased the expression of γ-H2AX as a result of promoting DNA damage (Fig. 1C).
Figure 1. BVAN08 inhibited DNA double-strand breaks repair induced by radiation. A: Comet image of HepG2 cells exposed to radiation. B: The quantification of DNA DSB as expressed as the comet tail moment. C: g-H2AX induced by radiation and participate in the repair process
BVAN08 combined irradiation degraded DNA-PKcs in HepG2 cells
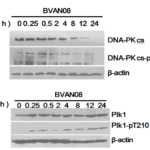
As the protein kinase activity of the DNA-PKcs and its autophosphorylation are critical for DBS repair via NHEJ (non-homologous end-joining), we check DNA-PKcs both in radiation and BVAN08 combination treatment. Radiation induced DNA-PKcs increased significantly, however, DNA-PKcs degraded in pretreated with BVAN08. Moreover, the phosphorylation of Thr-2609 was also reduced which indicating BVAN08 inhibits irradiation-induced DNA double-strand break repair (Fig. 2A). Plk1 is involved in the regulation of DNA damage detection in G2 and M phases, which is necessary for re-entry of mitotic phase after DNA damage recovery. Plk1 rapidly increase post irradiation suggested Plk1 involved in the early response to DNA damage which related to the regulation of mitotic progression after cell cycle arrest due to the action of BVAN08. Its phosphorylation of Thr-210 site also gradually increased (Fig. 2B).
BVAN08 combined irradiation activate cell cycle checkpoint in HepG2 cells
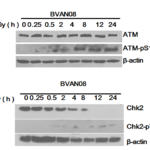
ATM plays an important role in repairing of ionizing radiation-induced DNA double-strand breaks, which can activate downstream Chk2 kinase. The activated Chk2 can phosphorylate p53 and CDC25 which will block the cell cycle. 60 μM BVAN08 pretreated HepG2 cells followed by radiation induced severe DNA damage and activation of the ATM pathway which lead to an increase in phosphorylated ATM (Fig. 3A). Moreover, phosphorylated Chk2 (Thr68 site) was activated by combine treatment BVAN08 with radiation (Fig. 3B).
Figure 2. DNA damage repair was inhibited by BVAN08. A. DNA-PKcs and phosphorylated DNA-PKcs / p-T2609 was inhibited by BVAN08. B. Plk1 and Phosphorylated Plk1 participated in DNA damage repair process induced by radiation
Figure 3. Cell cycle checkpoint was activated by radiation and BVAN08. A. Phosphorylated ATM was activated by combine treatment of radiation and BVAN08. B. Phosphorylated Chk2 was activated by combine treatment of radiation and BVAN08.
Discussion
Chemotherapeutic drugs kill cancer cells by inducing DNA damage. Effective DNA repair capability is an important intrinsic factor that determines the susceptibility of cancer cells to DNA damage. Ionizing radiation can induce many types of cell damage, including DSB repair and damage signal responses. The assembly and activation of the DSB repair system occurs in situ immediately after DNA fragmentation [9]. DNA double strand breaks were detected by neutral single cell electrophoresis. The results indicating irradiation with 4 Gy 60Co γ-rays could induce DNA damage in HepG2 cells and repaired after 4 hours. When being pre-treated with 40 μM BVAN08 for 12 h, significant cell tailing still be detected after 8 h of irradiation. Therefore, our result indicating BVAN08 inhibits DNA double strand breaks repair induced by irradiation.
Intracellular H2AX is phosphorylated to γ-H2AX at position 139 after eukaryotic DSBs are produced. Thus, γ-H2AX is considered as a molecular marker of DNA double-strand breaks. It has been reported in the literature that spindle nuclei activate DNA-PK after DSBs, thereby phosphorylating H2AX into γ-H2AX in the spindle [10]. The effect of combination BVAN08 with radiation on γ-H2AX in HepG2 cells was detected by Western blot. The results showed that γ-H2AX induced by BVAN08 with radiation in HepG2 cells, indicating BVAN08 promoted DNA damage and inhibited its damage repair process.
DNA-PK is an important DNA repair gene that can recognize and transmit DNA damage signal. Then the protein kinase is activated in order to phosphorylate DNA repair protein to start DNA strand break repair [11]. The results of western blot showed DNA-PKcs in BVAN08 cells was significantly decreased along with threonine at 2609 phosphorylation which indicated BVAN08 inhibited radiation-induced DNA double-strand break repair. Meanwhile, DNA-PKcs-deficient cells are sensitive than wild-type cells after ionizing radiation [12]. Therefore, the inhibition of DNA damage repair reaction by BVAN08 increased cell radiation sensitization effect.
Ionizing radiation-induced cell cycle arrest is the adaptive response of body cells to external stimuli. G2 arrest prevents the chromosomal abnormalities from mitosis during M phase to ensure the integrity and stability of the cell genome [13]. Plk1 is a highly conserved serine / threonine protein kinase that plays a very important role in the G2-M transition. Plk1 contains a T-loop domain in which T210 can be phosphorylated, which has kinase activity. It has been reported that HeLa cells overexpress Plk1can significantly increased G2 / M phase ratio [14]. Plk1 is involved in the regulation of DNA damage detection in G2 and M phases. Moreover, Plk1 is necessary to re-enter the mitotic phase after DNA damage recover. The changes of Plk1 in irradiated cell was increased rapidly after radiation suggesting Plk1 may be involved in the early response to DNA damage. The phosphorylation of cell cycle protein which regulated cell mitosis process also increased as the effect of BVAN08.
Mammalian cell cycle check point detection kinases include ATM and ATR. ATM can phosphorylate a range of substrates when DNA damaged, including Chk2, p53, MDM-2, etc. [15]. The activated Chk2 phosphorylate Cdc25C, thereby inhibiting the activity of Cyclin B-CDK1 complex which can arrest cell cycle in G2 phase [16]. ATM phosphorylates H2AX at the site of the DSB damage in the nucleus. However, ATM autophosphorylation occurs in the cytoplasm. When cells occurs DSBs in the cellular DNA, not only H2AX serine phosphorylation at position 139, but also ATM serine phosphorylate at position 1981, DNA-PKcs threonine phosphorylate at position 2609, Chk2 is phosphorylated at position 68 of threonine [17]. Our results showed that there was no significant change of ATM in BVAN08 cells treated with radiation. However, the phosphorylation of ATM at position 1981was increased followed by irradiation. It has been reported that the ATM is closely related to the radiosensitivity of glioma[18]. The radiation sensitizing effect of BVAN08 is related to the increase of phosphorylated ATM protein. Cell cycle checkpoint kinases, Chk1 and Chk2, play an important role in the regulation of G2 phase after injury. Inhibition of Chk1 and Chk2 can reduce cell cycle arrest post-irradiation and increase the radiosensitivity [19]. Chk2, in addition to being phosphorylated by both ATM and ATR, can also be phosphorylated by DNA-PK in vitro. Moreover, DNA-PK is involved in the activation of Chk2 in DNA damage response in vivo [20]. Our results showed combined effect of BVAN08 and radiation decreased Chk2 and increased the phosphorylation (Thr68) in BVAN08 cells, which may result in a continuous DNA damage signal activation ATM / Chk2.
Our study indicated BVAN08 not only can induce DNA double-strand breaks, but also inhibit DNA-chain-breakage repair induced by radiation. BVAN08 inhibited DNA-PKcs and activated ATM / Chk2 signaling pathways, enhance radiosensitivity of HepG2. Therefore, BVAN08 has the potential for developing into an effective radiation sensitizing drug.
Conflict of interest
There are no competing interests to declare.
Acknowledgments
This work was supported by Chinese National Natural Science Foundation (Grants No. 81372925, 31370843, 81530085) and the foundation of Jinzhou medical school.
References
- Takahashi A. and Ohnishi, T. Does gammaH2AX foci formation depend on the presence of DNA double strand breaks. Cancer Lett. 2005; 229(2): 171-179.
- Kozlov SV, Graham ME, Peng C, Chen P, Robinson PJ, Lavin MF. Involvement of novel autophosphorylation sites in ATM activation. EMBO. 2006; 25(15): 3504-3514.
- Mailand N, Falck J, Lukas C, Syljuâsen RG, Welcker M, Bartek J, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000; 288(5470): 1425-1429.
- Petronczki M, Lenart P, Peters J M. Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev Cell. 2008; 14(5): 646-659.
- Lowery DM, Lim Chem, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005; 24(2): 248-259.
- Bo Zhang, Lantao Liu, Hua Guan, Hui Wang, Zhen Zhang, Pingkun Zhou. HepG2 cell cycle related gene transcriptional profiles are altered by a novel vanillin derivative BVAN08. J Med Discov. 2017; 2(3): 58-62.
- Bo Zhang, Bo Huang, Hua Guan, Shimeng Zhang, Qinzhi Xu, Xingpeng He, et al. Proteomic profiling revealed the functional networks associated with mitotic catastrophe of HepG2 hepatoma cells induced by 6-bromine-5-hydroxy-4-methoxybenzaldehyde. Toxicol Appl Pharmacol. 2011;252(3):307-17
- Yan YQ, Zhang Bo, Wang L, Xie YH, Peng T, Bai B, et al. Induction of apoptosis and autophagic cell death by the vanillin derivative 6-bromine-5-hydroxy-4- methoxybenzaldehyde is accompanied by the cleavage of DNA-PKcs and rapid destruction of c-Myc oncoprotein in HepG2 cells. Cancer Letters. 2007; 252: 280-289
- Phillips ER, McKinnon PJ. DNA Double-strand break repair and development. Oncogene.2007; 26: 7799-7812.
- Chen H, Zeng X, Gao C, Ming P, Zhang J, Guo C, et al. A new arylbenzofuran derivative functions as an anti-tumour agent by inducing DNA damage and inhibiting PARP activity. Science Report. 2015; 4(5):10893.
- Yang L, Yang X, Tang Y, Zhang D, Zhu L, Wang S, et al. Inhibition of DNA‑PK activity sensitizes A549 cells to X‑ray irradiation by inducing the ATM‑dependent DNA damage response. Mol Med Rep. 2018;17(6):7545-7552.
- Tang S, Liu B, Liu M, Li Z, Liu J, Wang H, et al. Ionizing radiation-induced growth in soft agar is associated with miR-21 upregulation in wild-type and DNA double strand break repair deficient cells. DNA Repair (Amst). 2019; 78:37-44.
- Jang YJ, Ma S, Terada Y, Erikson RL. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J Biol Chem. 2002; 277(46): 44115-44120.
- Chen Y, Sanchez Y. Chk1 in the DNA damage response conserved roles yeasts to mammals. DNA Repair. 2004; 3(8-9): 1052-1032.
- Gaul L, Mandl-Weber S, Baumann P, Emmerich B, Schmidmaier R. Bendamustine induces G2 cell cycle arrest and apoptosis in myeloma cells: the roal of ATM-Chk2-Cdc25A and ATM-p53-p21-pathway. J Cancer Res Clin Oncol. 2008; 134(2): 245-53.
- Tian Bao-Lei, Sun Zhi-Xian. Promyelocytic leukemia protein and genomic stability. Chin J Biochem Mol Biol. 2006; 22(5): 349-354.
- Uematsu N, Weterings E, Yano K, Morotomi-Yano K, Jakob B, Taucher-Scholz G, et al. Autophosphorylation of DNA-PKcs regulates its dynamics at DNAdouble-strand breaks. Cell Biol. 2007; 177(2): 219-229.
- Tao Sheng-zhong, Niu Guang-ming, Lei Ting. Relationship between ataxia-telangiectaxia mutated protein expression and radiosensitivity in human gliomas. Chinese Journal of Experimental Surgery. 2009; 26(5): 35-39.
- Serrano MA, Li Z, Dangeti M, Musich PR, Patrick S, Roginskaya M et al. DNA-PK, ATM and ATR collaboratively regulate p53-RPA interaction to facilitate homologous recombination DNA repair. Oncogene. 2013; 32(19):2452-62.
- Wang YX, Zhu SC, Feng W, Li J, Su JW, Li R. Effect on retardation of G2/M phase in esophageal carcinoma cells transfected with Chk1 and Chk2 sh RNA after irradiation. Chin J Oncol. 2006; 28(8): 572-577.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/