J Med Discov (2018); 3(2):jmd18021; DOI:10.24262/jmd.3.2.18021; Received April 27th, 2018, Revised May 03rd, 2018, Accepted May 07th, 2018, Published May 17th, 2018.
Effects of tanshinone ⅡA on sodium channel currents in rat ventricular myocytes
Hongxin Ma1,2,3, Yi Kuang1,2,3, Maomao Sha1,2,3, Li Zou1,2,3, Xiuxiu Wang1,2,3, Wei Qian1,2,3, Jingna Ren1,2,3, Benlong Rao1,2,3, Zhengxin Xu1,2,3,4
1Department of Pharmacology, Medical School, Yangzhou University, Yangzhou 225001, PR China
2Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou 225001, PR China
3Jingsu Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment of Senile Diseases, Yangzhou 225001, PR China
4Jiangsu Key Laboratory of Experimental & Translational Non-coding RNA Research,Yangzhou 225009, PR China
* Correspondence: Zhengxin Xu, MD, PhD, Phone: +86-514-87978877 Fax: +86-514-87341733,Email: xuzhengxin405@126. com
Abstract
BACKGROUND: The function of the heart can be affected by the physiological and pathological changes of various ion channels in myocardial cell membrane, especially voltage-gated ion channels. This study is to explore the effects of tanshinone IIA on INa and kinetic characteristics of channels in ventricular myocytes of SD rats.
METHODS: Firstly, cut rat heart rapidly after anesthesia. Secondly, obtain single ventricular myocyte by reverse perfusion of Langendorff aorta. Then, record sodium currents by whole-cell patch clamp technique and add tanshinone IIA in the way of extracellular fluid elution and we explored the effects of different concentrations of tanshinone ⅡA (5,10,20,40,80,160 μM) on INa Finally, analyse the changes of kinetic characteristics of the sodium channel.
RESULTS: When the concentration of tanshinone ⅡA was 5 and 10 μM, there was no significant effect on INa, while the concentration was more than 20 μM, the inhibitory effect on INa was enhanced with the increase of concentration, among which, 20 and 80 μM tanshinone IIA reduced INa-Peak from (-110.61±6.28) pA/pF to (-87.28±5.76) pA/pF and (-54.53±3.15 ) pA/pF (P<0.01, n=6). I-V curve of INa shifted up significantly which affected by tanshinone IIA. However, the trajectory of I-V curve, Vac, VPeak, and Vrev have not been changed. Tanshinone IIA had no significant effect on the steady-state activation curve of INa. In addition, the steady-state inactivation curve of INa shifted to the left significantly, that is, moving to the hyperpolarization. Moreover, the recovery curve of INa shifted to the right.
CONCLUSIONS: Tanshinone IIA inhibited INa in a concentration-dependent manner and affected the kinetic characteristics of sodium channel.
Keywords: cardiac muscle cell, sodium channel, tanshinone IIA, patch clamp
Introduction
In recent years, with the improvement of people’s living conditions, the incidence and mortality of cardiovascular diseases such as arrhythmia, myocardial infarction and sudden death have increased rapidly. The prevention and treatment of these diseases has become one of the key points in the current medical work. As far as arrhythmia is concerned, although there are many types of arrhythmias, and the etiology and pathogenesis are complicated [1], but the process of disease must involve the changes of the excitability and electrophysiology of cardiac myocytes [2,3]. Therefore, it is very important to understand the process of change and the cause of change, and to find effective drugs for the treatment of these diseases.
The traditional Chinese medicine Danshen is often used in the prescriptions for the treatment of cardiovascular diseases, and it is the dry root and rhizome of Salvia miltiorrhiza Bge [4]. The function of Danshen are activating blood circulation, regulating meridians, dispelling blood stasis, relieving pain, cooling blood, eliminating carbuncle, eliminating irritation and tranquilizing the mind[5]. Qu et al. [6] found that Guanxin Danshen dropping pills could effectively reduce the score of blood stasis syndrome and serum sICAM-1 / sVCAM-1 level in patients with chronic stable angina pectoris and blood stasis syndrome. Wang [7] also expounded the unique advantages of Danshen and its modern preparations in the prevention and treatment of ischemic cardiovascular disease. The main liposoluble component of Danshen is tanshinone, which belongs to the flavonoids of fat-soluble phenanthraquinone, from which more than 10 tanshinone monomers, such as tanshinone Ⅰ, tanshinone ⅡA, tanshinone ⅡB, cryptotanshinone and isocryptotanshinone, were obtained. And it was proved by clinical trial that it was a new drug for treating coronary heart disease because of its remarkable effect and little side effect in the treatment of angina pectoris [8]. Tanshinone ⅡA is a diterpenoid compound of tanshinone type, which has a wide range of physiological activities, including anti-inflammatory, improving blood circulation, anti-tumor, anti-oxidation, protecting liver and improving liver function, providing protection of spinal cord, renal tubule and renal interstitial injury [9], inhibiting left ventricular hypertrophy and ischemic arrhythmia [10]. The common dosage form for it is sodium tanshinone ⅡA sulfonate injection.
Arrhythmia is a great threat to human life, including LQTS, SQTS, BRS, CPVT and ARVD / C, etc. In addition, arrhythmia caused by the disorder of ion channels in the myocardial cell membrane are called cardiac ion channel diseases [11]. Many ion channels on the membrane, especially voltage-gated ion channels, such as sodium, potassium and calcium channels, are abnormal in opening and closing, which can lead to changes in ion currents across myocardial membranes, inducing arrhythmias and even sudden death [3]. Therefore , focusing on the function of ion channel of myocardial cells has become a new target to treat arrhythmia by electrophysiological technique. It has been reported that the changes of calmodulin and ion channel protein gene expression in rabbit cardiomyocytes may be the important mechanism of ventricular arrhythmias in acute myocardial infarction. Tanshinone ⅡA can significantly reduce the incidence of ventricular arrhythmias, and the molecular mechanism of reducing myocardial infarction area may be related to the changes of calmodulin and ion channel protein gene expression [12], prevent and treat AF by improving atrial electrical remodeling induced by down-regulated expression of Kv1.5 in rats with AF [13], reduce the density of IKr and IKs in hypertrophic cardiomyocytes [14] and inhibit LTCC and decrease the current density of it in a concentration-dependent manner [15]. However, the effect of tanshinone ⅡA on Nav in cardiomyocytes is unclear.
In this study, we will study whether tanshinone ⅡA, an effective component of traditional Chinese medicine Danshen, has an effect on myocardial sodium current and explore the possible mechanism of its anti-arrhythmic effect.
Materials and Methods
Animals
SD rats, 200-300 g, which were provided by Yangzhou University Center for Comparative Medicine, and male and female are unlimited.
Medicines
Tanshinone ⅡA (Chengdu Purse Biotechnology Co., Ltd.) , Collagenase II(Worthington Company), Taurine, CsCl, TEACl, MgATP and HEPES(Sigma Co.), Heparin sodiumand Glucose(Solarbio Co.), EGTA(Fluka Co.), BSA(ICN Co.), TTX(Shanghai Kelamar Reagent Co., Ltd.), L- potassium glutamate (Vetec Co.), all of the NaCl, KCl, CaCl2, KOH, NaOH, CsOH·H2O, MgCl2·6H2O, NaH2PO4·2H2O and L-glutamic acid were pure product made in china.
Preparation of reagent
Calcium-free Tyrode’s solution(mmol/L): NaCl 136.0, KCl 5.5, NaH2PO4 0.3, MgCl2 1.0, HEPES 5.0, Glucose 10.9. The pH was adjusted to 7.35 ~ 7.40 with NaOH.
Tyrode’s solution: Adding CaCl2 into calcium-free Tyrode’s solution to the final concentration of 1.8 mmol/ L.
Enzyme solution: Adding 0.4 g/L collagenase II, 1 g/L BSA, 0.4 g/L Taurine, 30.0 moL CaCl2 into calcium-free Tyrode’s solution.
KB solution: L-glutamic acid 70.0, Taurine 10.0, KCl 25.0, EGTA 0.5, Glucose 11.0 HEPES 5.0, KOH 89.0. The pH was adjusted to 7.35 ~ 7.40 by KOH.
Electrode liquid(mmol/L): CsCl 133.0, NaCl 5.0, TEACl 10.0, EGTA 10.0, HEPES 5.0, MgATP 5.0. The pH was adjusted to 7.30 by CsOH.
Extracellular Fluid(mmol/L): NaCl 135.0, CsCl 5.4, MgCl2 1.0, CaCl2 1.8, HEPES 5.0, Glucose 10.0, CdCl2 0.1. The pH was adjusted to 7.40 by NaOH.
Tetrodotoxin (TTX): 50 µmol/L(µM) [16].
Tanshinone ⅡA: Cryopreservation of 100 mmol/L(mM) mother liquor dissolved in ethanol [17], and the corresponding extracellular solution was used to dilute the mother liquor making the final concentration were 5、10、20、40、80、160 µmol/L(µM).
Stimulus package
INa single stimulation: In the voltage clamp mode, set holding potential at -80 mV, give an square wave stimulation at -20 mV, 30 ms duration.
INa I-V curves: In the voltage clamp mode, set holding potential at -80 mV, give the square wave string stimulation at -70~90 mV, and the step was 5 mV, duration was 30 ms, the frequency was 0.5 Hz. The I-V curves of INa were obtained by using the INa-Peak as the y axis and the membrane potential as the x axis. The activation current stimulation scheme was as follows: set holding potential at -80 mV, give the square wave string stimulation at -80~10 mV, and the step was 5 mV, duration was 30 ms, the frequency was 0.5 Hz .
INa activation curve: The original data of the activation curve of INa was transformed from the partial data of the I-V curve. The figure was completed by taking the relative conductance G/Gmax(G=I/(V-Vrev)) as the y axis and the membrane potential as the x axis. The steady-state activation curve was obtained by fitting the Boltzmann equation G/Gmax= 1/{1+exp [(V1/2-ac-V)/ k ] }. Vrev is the voltage at which the I-V curve intersects the x axis (that is, the voltage from the inward current to the outgoing current, that is, the voltage at y=0), and k is the slope factor.
INa inactivation curve: In voltage clamp mode, the method of double pulse stimulation was used. Set the holding potential at -80 mV and conditional pulse of depolarization from -140 mV to 20 mV, the step was 10 mV, duration was 50 ms. Then start the repolarization to -30 mV, lasting 25 ms. The figure was completed by setting the relative current I/Imax as y axis and the membrane potential as x axis. The steady-state inactivation curve was obtained by fitting the Boltzmann equation I/Imax =1/{ 1 + exp [ (V-V1/2-in)/ k ] }, k is the slope factor.
INa recovery curve after inactivation: In voltage clamp mode, the method of double pulse stimulation was used. The stimulation of depolarization to -30 mV lasted for 30 ms. After returning to holding potential for 5 ms, a series of depolarization at -30 mV for 30 ms was given. And the interval between the two pulses was increased by a range of 5 ms, with a total of 15 pulses. Then Fit with One-phase association exponential equation I/Ipre=1−exp(−t/τ), I/Ipre is the ratio of the latter pulse to the previous pulse current, t is the interval between two pulses and τ is the recovery time when the channel is reactivated to the maximum current.
Acute isolation of ventricular myocytes from rats
All procedures were approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicine of Yangzhou University. The isolation of rat ventricular myocytes was improved with reference to relevant report [16]. In short, 2 000 IU kg-1 heparin sodiumand was injected intraperitoneally for 10 min, and then 2% pentobarbital sodium (40 mg·kg–1) was injected intraperitoneally. After anesthesia, the chest was opened quickly and the heart was cut. Then the free heart was cleaned at 4℃ calcium-free Tyrode’s solution, and the aorta was inserted and fixed on the modified Langendorff perfusion frame after simple pruning. The method comprises the following steps of : Firstly , the heart was resuscitated for 5 seconds by the infusion of Tyrode’s solution and the internal blood stain was removed. Secondly, the heart was perfused for 5 ~ 10 min with calcium-free Tyrode’s solution. Thirdly, perfusing the heart with enzyme solution for 20 ~ 30 min at a speed of about 4 ~ 6 ml/min, until the heart began to swell, transparent, and the droplets became turbid, and the long rod cells appeared at the time of microscopic examination. Finally, the digestion was stopped quickly, and the KB solution was injected into the aortic orifice with 2.5 ml volume syringe to further terminate the digestion and protect the cells. The whole perfusion process maintained constant temperature of 37 ℃ and total oxygen. After the digestion was terminated, the ventricular tissue was cut off quickly and shredded in KB solution. After filtration with 120 mesh screen, filtrate was placed at room temperature for 10 min, then the supernatant was discarded. The precipitate was cleaned with KB solution, and it was repeated for 3 times. Then the cell suspension was filled with total oxygen for 30 min, and then placed 2~3 h standby away from the light.
Electrode and other experimental conditions
The glass microelectrodes used in the experiment were made by a programmed P-97 microelectrode horizontal drawing instrument. An electrode with a tip diameter of 1 ~ 2 µm was made by drawing in five steps, and the electrode liquid was injected at the end of the electrode. The parameters are that the resistance of the electrode is kept at 2~4 MΩ after entering the extracellular Fluid, and the series resistance compensation was 20%~40%.
Whole-cell patch clamp recording
The whole-cell patch clamp recording was done with reference to relevant report [17].
Statistical methods
PatchMaster Software was used to recorded current. Origin7.0 software was used to transform the experimental data and graphpadprism6.1 was used to analyze and fit the data. SPSS 16.0 software was used for statistical processing of experimental data, and all experimental data were expressed as`x ± S, and the standard of statistical test was P<0.05.
Results
Effects of tanshinone ⅡA on INa in rat ventricular myocytes
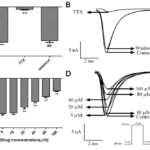
The sodium current was recorded in accordance with the current stimulation scheme of appendix 2.1. First of all, 50 μM TTX [18], the specific blocker of Nav, was added to the extracellular fluid, and the current was recorded under this condition. And then, the drug was eluted with extracellular fluid and the current was recorded again. As shown in Fig. 1A and B, the recorded current was an inward current, and the current was significantly suppressed under the influence of 50 μM TTX, and recovered after elution. It can be determined that the recorded current was a sodium current. Then the effects of different concentrations of tanshinone ⅡA (5,10,20,40,80,160 μM) which was dissoluted by alcohol [19] on INa in ventricular myocytes were observed after 10 min administration, as shown in Fig. 1C and D, when the concentration of tanshinone ⅡA was 5 and 10 μM, there was no significant effect on INa, while the concentration was more than 20 μM, the inhibitory effect on INa was enhanced with the increase of concentration.
Figure 1. Effects of drugs on INa. A. The change of INa-peak which was affected by 50 μM TTX and eluted by extracellular fluid. B. The single stimulus primary current of INa which was affected by 50 μM TTX and eluted by extracellular fluid. C. The changes of INa-Peak induced by tanshinone ⅡA at 5, 10, 20, 40, 80 and 160 μM. D. The primary current of INa under the influence of different concentrations of tanshinone ⅡA. Compared with control group **P<0.01, n=6 and compared with 50 μM TTX, ## P<0.01, n=6.
Effects of tanshinone ⅡA on the I-V curve of INa
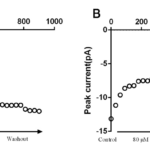
The INa (Fig. 2A) in the presence or absence of tanshinone IIA was recorded by using the stimulation scheme described in appendix 2.2, and the I-V curve of INa was drawn (Fig. 2B). It can be seen from the figure that tanshinone IIA can significantly move up the I-V curve of INa, and 20 and 80 μM tanshinone IIA reduced INa-Peak from (-110.61±6.28) pA/pF to (-87.28±5.76) pA/pF and (-54.53±3.15 ) pA/pF respectively(P <0.01,n= 6). However, the trajectory of I-V curve, Vac, VPeak and Vrev did not change.
Figure 2. Effects of tanshinone ⅡA on the activation process of INa. A. The effect of 20 and 80 μM tanshinone IIA on the primary activation current of INa. B. The effect of 20 and 80 μM tanshinone ⅡA on the I-V curve of INa, compared with control group, P <0.01, n= 6. C. The effect of 20 and 80 μM tanshinone IIA on the steady-state activation curve of INa, compared with control group, P >0.05, n= 6.
Effects of tanshinone ⅡA on the activation process of INa
The original data of the steady-state activation curve of INa were transformed from part of the data of I-V curve, and the steady-state activation curve was obtained according to the fitting method of appendix 2.3(Fig. 2C). As shown in the figure, tanshinone IIA had no significant effect on the steady-state activation curve of INa. V1/2-ac changed from (-43.85±8.07)mV to (-43.95±2.44)mV and (-43.99±0.79)mV.
Effects of tanshinone ⅡA on the inactivation process of INa.
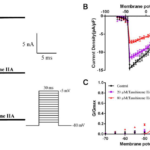
The steady-state inactivation current of INa in the presence or absence of tanshinone IIA was recorded by the appendix 2.4 stimulus (Fig. 3A). And the steady-state inactivation curve was obtained by fitting the equation(Fig. 3B). It can be seen from the figure that tanshinone IIA can make the steady-state inactivation curve of INa shift significantly to the left, that is, to the direction of hyperpolarization. 20 and 80 μM tanshinone IIA made V1/2-in from (-52.15±0.96)mV to (-64.87±0.99)mV and (-75.71±0.75)mV(P<0.05, n=6), and the value of k changed from (6.41±0.85) to (7.92±0.88) and (10.92±0.66)(P <0.05, n= 6). Therefore, tanshinone IIA can obviously change the inactivation kinetics of INa and accelerate the inactivation of sodium channel.
Figure 3. Effects of tanshinone ⅡA on the inactivation process of INa. A. The effect of 20 and 80 μM tanshinone IIA on the primary inactivation current of INa. B. The effect of 20 and 80 μM tanshinone IIA on the steady-state inactivation curve of INa, compared with control group, P<0.05, n= 6.
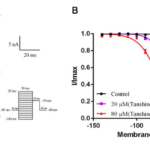
Figure 4. Effects of tanshinone ⅡA on the recovery process of INa. A. The effect of 20 and 80 μM tanshinone IIA on the primary recovery current of INa. B. The effect of 20 and 80 μM tanshinone IIA on the recovery curve of INa, compared with control group, P<0.05, n= 6.
Effects of tanshinone ⅡA on the recovery process of INa.
The recovery currents of the channel in the presence or absence of tanshinone IIA were recorded according to the appendix 2.5 stimulus(Fig. 4A), and the recovery curve of INa was obtained by fitting the equation(Fig. 4B). As shown in the figure that tanshinone IIA can make the recovery curve of INa shift to the right, 20 and 80 μM tanshinone IIA made τ from (11.52±1.18)ms to (20.84±0.99)ms and (30.81±1.01)ms (P<0.05, n=6). It is concluded that tanshinone IIA can change the recovery dynamic characteristics of the sodium channel after inactivation and prolong the recovery time of INa from inactivation state to activation state.
Discussion
An effective way to explore the function of ion channels is the patch clamp technique, which was founded by Neher and Sakmann in the Mapper Biophysical Laboratory in Germany in 1976 [20]. It is a technique that reflects the activity of a single (or multiple) ion channel molecule on a cell membrane, based on the principle that pre-treated glass microelectrodes are used to contact the cell and form a blocking state of the kilomega ohm(GΩ) or more impedance. At this time, the small area of the membrane in contact with the tip of the electrode is electrically separated from the other structures around it, so the ionic channel current on the membrane can be recorded on the basis of the fixed membrane potential [21]. With the development of this technology, the characteristics and physiological meaning of various ion channels in cells have been recognized and understood. For example, the sodium ion current in cardiomyocytes is an inward current, which is mainly involved in the depolarization process of the cell AP 0 phase, which affects the Rm of the cell, and then affects the excitability of the cardiomyocytes and the ECG waveform [2].
Sodium channels are channels that selectively allow sodium ions to through the cell membrane, including VGSC (Nav, also known as voltage-dependent sodium channels), ENaC, ASIC, and Nax channels, and Nav is the most typical [22]. Nav is subdivided into nine sub-types according to the functional α subunit of channel, Nav1.1-1.9, among them, Nav1.5 is mainly distributed on myocardial cell membrane, which has important significance for the normal exertion of cardiomyocyte and cardiac physiological function. Therefore, it is a new research direction to explore the therapeutic effect of drugs on heart disease for Nav1.5 target in recent years.
Therefore, the aim of this study was to explore the possible mechanism of anti-arrhythmic effect of tanshinone IIA, an effective component of traditional Chinese medicine Danshen, at the ion channel level. In introduction section, we learned that tanshinone IIA can affect LTCC, Kv1.5, IKs and IKr. In addition, Ao Ri-Ge-le[23] et.al found tanshinone IIA attenuated painful diabetic neuropathy by effecting VGSCs activities and expressions in dorsal root ganglion. Thus, in order to observe the effects of tanshinone IIA on INa in myocytes, we selected isolated rat ventricular myocytes as the research object, because the physiological characteristics of atrial and ventricular myocytes are different [17]. The whole-cell patch clamp technique was used to record INa, and by collecting and analysing the data, we can further understand the changes of the related kinetic processes of sodium channels under the influence of tanshinone IIA. We found that tanshinone IIA inhibited INa in SD rat ventricular myocytes in a dose-dependent manner. As for channel dynamics, tanshinone IIA could delay the activation, accelerate the inactivation, and slow down the recovery after inactivation of channels.
However, this study only focused on the cell level in vitro. The further research on the electrophysiological characteristics of isolated heart and even in vivo, the characteristics of each channel and the safety of drugs need to be improved. Besides Nav1.5, there are many other ion channels in ventricular myocytes, such as LTCC, Kv, etc, and the interaction between channels and the expression of channel proteins should also be taken into account [12]. Moreover, Tetrodotoxin (TTX) is recognized as a sodium channel blocker, However, the blocking effect and intensity of TTX are different due to the large number of sodium channel types and the different structural and functional characteristics of different sub-types. As Nav1.7, which is mainly distributed in peripheral nerves, is highly sensitive to TTX [24], while Nav1.5 is certainly resistant to it [22]. Therefore, searching for a more suitable blocker for this experiment should be included in the next research plan.
There is still a lot of work to be done. It is believed that the effects of tanshinone IIA in the treatment of arrhythmia will be well explained at the molecular and electrophysiological level, and further explore the possible mechanism of Salvia miltiorrhiza to exert antiarrhythmic effect.
Conflict of interest
None
Acknowledgments
We acknowledge National Natural Science Foundation of China (Grant No. 31071171) and the State Key Laboratory of Genetic Engineering program (Grant No. SKLGE-1405) for supporting our research.
References
1. Gao Y Y, Li Y X, Ma Y X, et al. Information on traditional Chinese Medicine. The effects of Hypericum acuminata serum on fast sodium channel currents in rat cardiomyocytes. 2017,34 / 4: 6-9
2. Hund TJ, Rudy Y. Biophysical Journal. Determinants of excitability in cardiac myocytes: mechanistic investigation of memory effect. 2000, 79(6):3095.
3. Garciaelias A, Benito B. International Journal of Molecular Sciences. Ion Channel Disorders and Sudden Cardiac Death. 2018, 19(3):692.
4. Chinese Pharmacopoeia. 1. 2015
5. Nanjing University of traditional Chinese Medicine. Great Dictionary of Chinese Medicine. 2006.
6. Qu H, Guo M, Chai H, et al. Journal of traditional Chinese Medicine. Effects of Guanxin Danshen dropping Pill on Blood stasis Syndrome score and Serum Adhesion Factor level in patients with chronic stable angina Pectoris and Blood stasis Syndrome. 2017,58: 394-397.
7. Wang J H. Shanghai Journal of Chinese Medicine. Ischemic cardiovascular disease used salvia miltiorrhiza, activating blood and nourishing blood without stimulation. 2017,004.
8. Liu S L. Chinese Medical Science. Progress in the protective mechanism of tanshinone against cardiovascular disease. 2015: 4: 32-34.
9. Jiang X R, Miao L, Wu X Y, et al. Chinese Contemporary Medicine. Advances in studies on the protective effect and mechanism of tanshinone ⅡA on cardiovascular system. 2014 , 21 (14) : 183 – 185 .
10. Chen F Y, Guo T, Zhang B K. Chinese Journal of traditional Chinese Medicine. Advances in the study of Cardiovascular Pharmacological effects of Tanshinone ⅡA. 2015,40: 1649-1653.
11. Hickey KT, Elzomor A. Aacn Adv Crit Care. Cardiac Channelopathies: Recognition, Treatment, Management. 2018:43-57.
12. Zhu L, Wang Z H. Journal of Huazhong University of Science and Technology (Medical Edition). Effects of tanshinone ⅡA on ventricular arrhythmias and ion channel protein gene expression after acute myocardial infarction in rabbits. 2014,4315: 501-505.
13. Yang J. Guiyang College of traditional Chinese Medicine. Effects of sodium tanshinone IIA-sulfonate on the expression of potassium channel Kv1.5 gene in atrial myocytes of rats with atrial fibrillation. 2012.
14. Tang Y, Sheng G T, Ge Y Z, et al. Chinese Journal of Pathophysiology. Effects of tanshinone ⅡA on delayed rectifier potassium channels in hypertrophic myocardium of guinea pigs. 2012,28: 234-238.
15. Huang J, Pei J H, Teng S Y, et al. Chinese Journal of Molecular Cardiology. Effects of tanshinone IIA on L-type calcium channel in rat cardiomyocytes. 2012,12: 106-109.
16. Xu H E , Tao J, Chen J, et al. Journal of Nanjing Medical University. Isolation of Ca2+ Tolerant Cardiomyocytes from Aadult Rats for Patch Clamp Studies. 2004.
17. Zheng X. Yangzhou University. Effects of osthole on ion channels in rat ventricular myocytes. 2016.
18. Wang G T, Bai Y, Fan X L, et al. South of the Five Ridges Journal of cardiovascular diseases. Effects of ranolazine on the sodium currents in guinea pig ventricular myocytes. 2017, 23 (3): 319-321.
19. Bébarová M, Matejovič P, Pásek M, et al. Acta Physiologica. Effect of ethanol on action potential and ionic membrane currents in rat ventricular myocytes. 2010, 200(4):301-314.
20. Neher E, Sakmann B. Nature. Single-channel currents recorded from membrane of denervated frog muscle fibres. 1976, 260(5554):799.
21. Liu Z W. Practical patch clamp technique. 2006.
22. Guan B C, Zhang H L, Li Z W. Science Press. Basic principles of cellular electrophysiology and patch clamp techniques. 2013.
23. Ri-Ge-Le A, Guo Z L, Wang Q, et al. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. Tanshinone IIA Improves Painful Diabetic Neuropathy by Suppressing the Expression and Activity of Voltage-Gated Sodium Channel in Rat Dorsal Root Ganglia. 2018.
24. Tsukamoto T, Chiba Y, Wakamori M, et al. British Journal of Pharmacology. Differential binding of tetrodotoxin and its derivatives to voltage-sensitive sodium channel subtypes (Nav1.1 to Nav1.7). 2017, 174(21).
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/