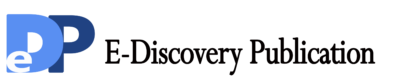J Med Discov (2018); 3(2):jmd18011; DOI:10.24262/jmd.3.2.18011; Received March 12nd, 2018, Revised May 02nd, 2018, Accepted May 10th, 2018, Published June 1st, 2018.
Biological screening of some novel pyrimidine compounds
Shipra Baluja1*, Sumitra Chanda2 and Kapil Bhesaniya1
1Department of Chemistry Saurashtra University, Rajkot-360005 (Gujarat), India
2Department of Bioscience Saurashtra University, Rajkot-360005 (Gujarat), India
* Correspondence: Shipra Baluja, Department of Chemistry Saurashtra University, Rajkot-360005 (Gujarat), Indiae. E-mail: shipra_baluja@rediffmail.com.
Abstract
Some novel pyrimidine compounds have been synthesized and their structural confirmation was done by different spectroscopic techniques such as FT-IR, NMR and MS. The biological screening of all the synthesized compounds is done in DMSO using agar well diffusion method. For the biological study, different Gram positive and Gram negative bacterial and fungal strains are used. It is observed that inhibition depends on strain, solvent and structure of compounds. In the present work, all the compounds were screened in DMSO solvent. The synthesized compounds have different moieties as well as substitutions. So, different strains are affected differently by different compounds.
Keywords: Pyrimidine compounds, DMSO, antimicrobial activity, agar well diffusion method
Introduction
The extensive use of antibiotics has led to the appearance of multidrug resistant microbial pathogens [1]. In recent years, multiple drug resistance has developed due to indiscriminate use of existing antimicrobial drugs in the treatment of infectious diseases. Further, antibiotics are sometimes associated with adverse effects on the host-like hypersensitivity. Therefore, there is a need to develop alternative antimicrobials drugs for the treatment of infectious diseases from other sources.
The biological activity spectrum of a compound represents the pharmacological effects, physiological and biochemical mechanisms of action, specific toxicity that can be revealed in compound’s interaction with biological system. Further, it describes the intrinsic properties of the compound, which depends on its structure.
Pyrimidines are always an attraction point for researchers because of its efficiency towards various pharmacological usages. These compounds are known to possess various biological activities [2-10]. Literature survey shows that various fused pyrimidine derivatives are known to exhibit anti-tubercular [11, 12], anti-proliferative [13, 14], anti-HIV [15, 16], anti-microbial [17], anti-analgesic [18], anti-inflammatory [19] and anti-malarial [20] activities. Compounds containing imidazo [2, 1-b] thiazole derivatives are also of great interest among medicinal chemists as these compounds have also been reported for a wide spectrum of other biological properties [21-26].
In the present paper, some novel pyrimidines compounds have been synthesized. The antimicrobial activities of synthesized compounds have been screened against some bacterial (both Gram positive and Gram negative) and fungal strains in DMSO. The results are reported as minimum inhibitory concentrations (MIC) and minimum bactericidal concentration (MBC) for all the synthesized compounds.
Materials and Methods
Drug synthesis
Synthesis of 2, 4-disubstituted pyrimidine derivatives (BKD-1 to BKD-12): In n-butanol, equimolar mixture of 2, 4-dichloropyrimidine (DCP), 4-((1H-1, 2, 4-triazol-1-yl) methyl) aniline (TMA) and 0.012 mole of N, N-diisopropyl ethyl amine was refluxed for 3 hr. The completion of reaction was confirmed by analytical thin layer chromatography (TLC) using as a 9.6 : 0.4 dichloromethane : methanol mobile phase. After completion of reaction, reaction mixture was cooled. The resulting solid was filtered, washed with cold water and dried under vacuum to give crude product.
This resulting product was refluxed for 3 hr with ethanolic solution of different aromatic amines (0.011 mol) using glacial acetic acid as catalyst. The completion of reaction was monitored using TLC (100% ethyl acetate + NH3 atmosphere as a mobile phase). After completion of reaction, the reaction mixture was cooled and the resulting solid was filtered, washed with cold ethanol and dried under vacuum to give crude product. The obtained crude product was purified by tituration with diethyl ether.
The reaction scheme is:
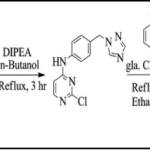
Synthesis of 2, 4-disubstituted pyrimidine derivatives (KDB-1 to KDB-9): Equimolar mixture of 2,4-dichloropyrimidine (DCP), 1-Naphthol (NTL) and 0.015 mole of Potassium carbonate (K2CO3) in DMF was refluxed for 4 hr. The completion of reaction was confirmed by analytical thin layer chromatography (TLC) using (7:3–Hexane: Ethyl acetate) as mobile phase. After completion of reaction, the reaction mixture was cooled and the resulting solid was filtered, washed with cold water and dried under vacuum to give crude product.
This resulting product (0.01 mol) was refluxed for 4-5 hr with ethanolic solution of different aromatic amines (0.012 mol) using hydrochloric acid as catalyst. The completion of reaction was confirmed by TLC using (7.5:2.5-Hexane: Ethyl acetate) mobile phase. After completion of reaction, the reaction mixture was cooled. The resulting solid was filtered, washed with cold ethanol and dried under vacuum to give crude product. The obtained crude product was purified by tituration with diethyl ether. The physical constants of all synthesized compounds are listed in Table 1.
Synthesis of substituted and fused pyrazolopyrimidines (TC-1 to TC-16):
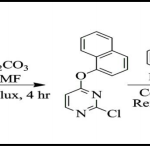
Ist Step: Synthesis of substituted acetoacetanilide derivatives (AAA) (Intermediate-I): Equimolar mixture of substituted aromatic amine (I), 1, 3-diketone (II) and catalytic amount of potassium hydroxide (KOH) in 1, 4-dioxane was refluxed for 4-5 hr. The progress of the reaction was monitored by TLC. After completion of reaction, reaction mixture was allowed to cool at room temperature and was poured into crushed ice. The obtained solid was filtered and was purified by titruation with hexane to get pure product (Intermediate-I).
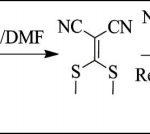
IInd Step: Synthesis of 5-Amino-3-(methylthio)- 1H-pyrazole-4-carbonitrile (Intermediate-II): A mixture of malano nitrile (0.01 m mol) and dry K2CO3 (0.012 m mol) were stirred in dry DMF at room temperature for 30 min., 0.02 mole carbon disulphide (CS2) was drop wise added in reaction mixture. Then, the reaction mixture was stirred for an additional 2.5 hr at same temperature. The reaction mixture was then cooled at 0-5 °C and dimethyl sulphate (0.02 mol) was added. The solution was stirred at room temperature for another 5-6 hr and was poured into crushed ice to give solid product. The resulting solid was filtered, washed with cold water and was dried under vacuum to give crude product.
This resulting crude product (0.01 mol) was refluxed with hydrazine hydrate (0.01 mol) for 30 min. in isopropyl alcohol (IPA). After completion of reaction, the reaction mixture was cooled and poured into crushed ice. The resulting solid was filtered, washed with water and dried under vacuum to give crude product (Intermediate-II). The obtained crude product was purified by tituration with hexane and used in next step without further purification.
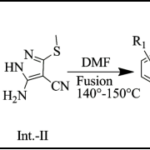
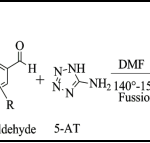
IIIrd Step: Synthesis of substituted and fused pyrazolo pyrimidines (TC-1 to TC-16): A mixture of Int.-I (0.01 mol), Int.-II (0.015 mol) and different substituted aldehyde (0.01 mol) was heated at 140-150 °C for 25-30 min. in presence 3-4 drops of DMF. The completion of reaction was confirmed by TLC. After completion of reaction, reaction mixture was allowed to cool at room temperature and poured into crushed ice. The resulting solid was filtered, washed with water and dried under vacuum which was then purified by tituration with methanol.
| BKD-series | |||||
|
Compound Code |
Substitution R |
M. F. |
M. Wt. (g/mol) |
Yield (%) | Rf value |
| BKD-1 | 4-Cl | C19H16ClN7 | 377.83 | 88 | 0.44 |
| BKD-2 | 4-CH3 | C20H19N7 | 357.41 | 92 | 0.46 |
| BKD-3 | 4-F | C19H16FN7 | 361.38 | 79 | 0.45 |
| BKD-4 | 3-CF3 | C20H16F3N7 | 411.38 | 89 | 0.48 |
| BKD-5 | 3-Cl, 4-F | C19H15ClFN7 | 395.82 | 78 | 0.41 |
| BKD-6 | 4-OCH 3 | C20H19N7O | 373.41 | 87 | 0.39 |
| BKD-7 | 3-Cl | C19H16ClN7 | 377.83 | 77 | 0.43 |
| BKD-8 | 3,4-dichloro | C19H15Cl2N7 | 412.28 | 89 | 0.40 |
| BKD-9 | 4-CF3 | C20H16F3N7 | 411.38 | 90 | 0.47 |
| BKD-10 | 3-CH3 | C20H19N7 | 357.41 | 92 | 0.47 |
| BKD-11 | 2-CH3 | C20H19N7 | 357.41 | 76 | 0.41 |
| BKD-12 | 2-F | C19H16FN7 | 361.38 | 74 | 0.40 |
| KDB-series | |||||
| KDB-1 | 4-Cl | C20H14ClN3O | 347.08 | 78 | 0.55 |
| KDB-2 | 4-CH3 | C21H17N3O | 327.38 | 71 | 0.52 |
| KDB-3 | 4-F | C20H14FN3O | 331.11 | 81 | 0.42 |
| KDB-4 | 3-CF3 | C21H14F3N3O | 381.35 | 86 | 0.58 |
| KDB-5 | 3-Cl, 4-F | C20H13ClFN3O | 365.79 | 78 | 0.54 |
| KDB-6 | 4-OCH3 | C21H17N3O2 | 343.38 | 77 | 0.50 |
| KDB-7 | 3, 4-dichloro | C20H13Cl2N3O | 382.24 | 80 | 0.54 |
| KDB-8 | 3-Cl | C20H14ClN3O | 347.80 | 77 | 0.52 |
| KDB-9 | 2-F | C20H14FN3O | 331.11 | 70 | 0.53 |
Table 1: Physical constants of 2,4-disubsstitutedpyrimidine derivatives.
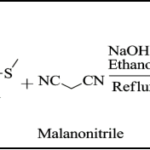
Synthesis of substituted and fused pyrazolopyrimidines (KD-1 to KD-12): An ethanolic solution of different substituted aromatic aldehyde (0.01 mol), Int.-II (0.015 mol) and malano nitrile (0.01 mol) was refluxed for 3-5 hr using sodium hydroxide as catalyst. The reaction mass was cooled and resulting solid was filtered, washed with cold ethanol and dried. The crude product was purified by tituration with diethyl ether.
The physical constants of all synthesized compounds are listed in Table 2.
| TC- series | ||||||||
| Comp. Code | Substitutions | M.F. | M. Wt. |
Yield (%) |
Rf value | |||
| R1 | R2 | R3 | ||||||
| TC-1 | 4-Cl | (CH3)2CH- | 4-Cl | C24H21Cl2N5OS | 498.43 | 68 | 0.61 | |
| TC-2 | 3-Cl | (CH3)2CH- | 4-Cl | C24H21Cl2N5OS | 498.43 | 59 | 0.63 | |
| TC-3 | 3,4-di Cl | (CH3)2CH- | 4-Cl | C24H20Cl3N5OS | 532.87 | 66 | 0.65 | |
| TC-4 | 3-Cl,4-F | (CH3)2CH- | 4-Cl | C24H20Cl2FN5OS | 516.42 | 56 | 0.69 | |
| TC-5 | 4-F | (CH3)2CH- | 4-Cl | C24H21ClFN5OS | 481.87 | 70 | 0.70 | |
| TC-6 | 4-CH3 | (CH3)2CH- | 4-Cl | C25H24ClN5OS | 477.14 | 65 | 0.70 | |
| TC-7 | 4-CF3 | (CH3)2CH- | 4-Cl | C25H21ClF3N5OS | 531.98 | 62 | 0.69 | |
| TC-8 | 4-Cl | (CH3)2CH- | 4-OCH3 | C25H24ClN5O2S | 493.01 | 62 | 0.68 | |
| TC-9 | 3,4-di Cl | (CH3)2CH- | 4-OCH3 | C25H23Cl2N5O2S | 527.09 | 59 | 0.66 | |
| TC-10 | 4-OCH3 | -CH3 | 4-OCH3 | C24H23N5O3S | 461.15 | 70 | 0.63 | |
| TC-11 | 4-Cl | -CH3 | 4-Cl | C22H17Cl2N5OS | 469.05 | 55 | 0.69 | |
| TC-12 | 4-F | -CH3 | 4-Cl | C22H17ClFN5OS | 453.08 | 66 | 0.64 | |
| TC-13 | 4-Br | -CH3 | 4-Cl | C22H17BrClN5OS | 514.83 | 69 | 0.65 | |
| TC-14 | 4-Br | -CH3 | 4-OCH3 | C23H20BrN5O2S | 509.04 | 67 | 0.63 | |
| TC-15 | 4-F | -CH3 | 4-OCH3 | C23H20FN5O2S | 449.13 | 70 | 0.64 | |
| TC-16 | 4-Cl | -CH3 | 4-OCH3 | C23H20ClN5O2S | 465.96 | 68 | 0.66 | |
| KD- series | ||||||||
| Compd. Code | Substitution R | M.F. | M. wt. |
Yield (%) |
Rf value | |||
| KD-1 | 4-OCH3 | C16H12N6OS | 336.37 | 72 | 0.41 | |||
| KD-2 | 3,4-di OCH3 | C17H14N6O2S | 366.40 | 71 | 0.40 | |||
| KD-3 | 4-Cl | C15H9ClN6S | 340.79 | 69 | 0.47 | |||
| KD-4 | 4-F | C15H9FN6S | 324.34 | 67 | 0.49 | |||
| KD-5 | -H | C15H10N6S | 306.35 | 69 | 0.48 | |||
| KD-6 | 3-Cl | C15H9ClN6S | 340.79 | 64 | 0.47 | |||
| KD-7 | 3-Br | C15H9BrN6S | 385.24 | 73 | 0.49 | |||
| KD-8 | 2,5-di-OCH3 | C17H14N6O2S | 366.40 | 72 | 0.39 | |||
| KD-9 | 3-OCH3 | C16H12N6OS | 336.37 | 71 | 0.46 | |||
| KD-10 | 4-N(CH3)2 | C17H15N7S | 349.41 | 69 | 0.48 | |||
| KD-11 | 4-CH3 | C16H12N6S | 320.37 | 67 | 0.51 | |||
| KD-12 | 3,4,5-tri OCH3 | C18H16N6O3S | 396.42 | 61 | 0.37 | |||
Table 2: Physical constants of Pyrazolopyrimidine derivatives
Synthesis of imidazothiazole derivatives (TP-1 to TP-9):
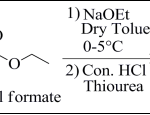
Ist Step: Synthesis of ethyl 2-aminothiazole-5- carboxylate (Int.-1): Equimolar mixture of ethyl chloro acetate and ethyl formate were added drop wise to a suspension of 0.01 mol solution of sodium ethoxide in dry toluene, maintained at a temperature between 0-5°C for 2 hr. Then, the reaction mixture was stirred at 0°C for another 2.5 hr. The contents were diluted with water and the layers were separated. The aqueous phase was acidified with concentrated hydrochloric acid. In this acidified solution, 0.013 mole of aqueous thio urea solution was added and the solution was refluxed for 2.5 hr. The completion of reaction was confirmed by TLC using (100% ethyl acetate) as mobile phase. After completion of reaction, the reaction mass was cooled and neutralized with sodium hydroxide solution. An amber colored solid was precipitated, which was filtered and dried to get desired product ethyl 2-amino thiazole-5-carboxylate.
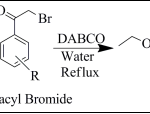
IInd Step: Synthesis of imidazothiazole derivatives (TP-1 to TP-9): A mixture of ethyl 2-amino thiazole-5-carboxylate (Int.-1) (0.01 mol), different substituted phenacyl bromide (0.012 mol) and 10 % aqueous solution of 1, 4-diazabicyclo [2.2.2]octane (DABCO) was refluxed for 1 hr. The completion of reaction was confirmed by TLC using (8:2-Hexane: Ethyl acetate) as mobile phase. After completion of reaction, the reaction mixture was cooled. The resulting solid was filtered, washed with cold water and dried. The obtained crude product was purified by tituration with mixture of methanol and ethyl acetate.
Synthesis of fused tetrazolopyrimidines derivatives (K-1 to K-14):
Synthesis of substituted aceto acetanilide derivatives (AAA) (Intermediate-I): Given above (of TC series).
Synthesis of fused tetrazolopyrimidines derivatives (K-1 to K-14): All the compounds (K-1 to K-14) were synthesized according to synthesis of fused pyrazolopyrimidines (TC series).
The physical constants of all synthesized compounds are listed in Table 4.
The formation of compounds was checked by TLC (Performed on aluminum coated TLC plates gel-G60 F254 and accomplished on 0.5-mm (E. Merck)). Visualization of spot was made with UV light (254 and 365 nm), an iodine vapor and other visualizing reagent. The melting point was determined in open capillary tubes and was uncorrected. IR spectra were recorded on KBr discs, using FT-IR, (Shimadzu spectrophotometer Model no.-8400). 1H-NMR spectra were taken on a Bruker AVANCE II 400. In all the cases, 1H NMR spectra were obtained in DMSO-d6 using TMS as an internal standard. The NMR signals are reported in δ ppm. Mass spectra were determined using direct inlet probe on a GCMS-QP-2010 mass spectrometer.
| Compound Code |
Substitution R |
M. F. |
M. Wt. (g/mol) |
Yield (%) | Rf value |
| TP-1 | 4-OCH3 | C15H14N2O3S | 302.35 | 64 | 0.41 |
| TP-2 | 4-Cl | C14H11ClN2O2S | 306.77 | 66 | 0.49 |
| TP-3 | 4-Br | C14H11BrN2O2S | 351.22 | 70 | 0.48 |
| TP-4 | 3,4-di-F | C14H10F2N2O2S | 308.30 | 61 | 0.49 |
| TP-5 | -H | C14H12N2O2S | 272.32 | 69 | 0.50 |
| TP-6 | 4-F | C14H11FN2O2S | 290.31 | 72 | 0.47 |
| TP-7 | 2,4-di-Cl | C14H10Cl2N2O2S | 341.21 | 69 | 0.46 |
| TP-8 | 4-NO2 | C14H11N3O4S | 317.32 | 67 | 0.43 |
| TP-9 | 4-CH3 | C15H14N2O2S | 286.35 | 63 | 0.48 |
Table 3: Physical constants of Imidazothiazole derivatives
Biological Screening
The antibacterial and antifungal activities of all synthesized compounds were studied in DMSO. All the synthesized compounds were recrystallized prior to use and DMSO was purified by standard method [27]. For all the compounds, agar well diffusion method was used.
Following Strains were used for the antimicrobial screening:
Gram positive bacteria:
- (I)Corynebacterium rubrumATCC14898 (CR)
- (II)Staphylococcus albusNCIM2178 (SAL)
(III)Staphylococcus aureus ATCC25923 (SA)
| Compound Code | Substitutions | M. F. |
M. Wt (g/mol) |
Yield (%) |
Rf value |
| R | |||||
| K-1 | -H | C21H19F3N6O | 428.41 | 51 | 0.44 |
| K-2 | 3,4-di-OCH3 | C23H23F3N6O3 | 488.46 | 59 | 0.39 |
| K-3 | 4-Cl | C21H18ClF3N6O | 462.86 | 62 | 0.42 |
| K-4 | 4-F | C21H18F4N6O | 446.40 | 63 | 0.44 |
| K-5 | 3-OH | C21H19F3N6O2 | 444.41 | 69 | 0.35 |
| K-6 | 4-OCH3 | C22H21F3N6O2 | 458.44 | 55 | 0.41 |
| K-7 | 3-Cl | C21H18ClF3N6O | 462.86 | 59 | 0.46 |
| K-8 | 4-OCHF2 | C22H19F5N6O2 | 494.42 | 54 | 0.40 |
| K-9 | 3-Br | C21H18BrF3N6O | 507.31 | 66 | 0.43 |
| K-10 | 4-CH3 | C22H21F3N6O | 442.44 | 67 | 0.45 |
| K-11 | 3-OCH3 | C22H21F3N6O2 | 458.44 | 68 | 0.42 |
| K-12 | 2,5-di-OCH3 | C23H23F3N6O3 | 488.46 | 66 | 0.43 |
| K-13 | 2- OCH3 | C22H21F3N6O2 | 458.44 | 64 | 0.41 |
| K-14 | 2-Cl | C21H18ClF3N6O | 462.86 | 52 | 0.46 |
Table 4: Physical constants of Tetrazolopyrimidine derivatives
Gram negative bacteria:
(I)Enterobacter aerogenes ATCC13048 (EA)
(II)Escherichia coli NCIM2931 (EC)
(III)Salmonella typhimurium ATCC23564 (ST)
Fungi(Yeast):
(I)Candida albicans ATCC2091 (CA)
(II)Candida neoformans NCIM3542 (CN)
(III)Candida glabrata NCIM3448 (CG)
All these strains were obtained from National Chemical Laboratory (NCL), Pune, India. The bacterial and fungal strains were maintained on nutrient agar and MGYP medium (Hi Media, India) respectively while E. coli were maintained on Luria medium (Hi Media, India) at 4°C and sub cultured before use.
MIC refers to the lowest concentration of the antimicrobial agent which is required for the inhibition of visible growth of the tested microorganism [28]. The antimicrobial activity of an agent is usually quantified by determining the MIC values which serve as a guide for treatment of most infections. MIC values were calculated using INT dye. The MBC is interpreted as the lowest concentration that can completely remove the microorganisms.
Preparation of solution of compounds for MIC and MBC study: All the compounds dissolved in DMSO were first diluted to highest concentration (20 mg ml-1) to be tested and then serial two-fold dilution was made in a concentration range from (0.156 to 20 mg ml-1).
Preparation of bacterial inocula for MIC and MBC study: The inocula of the test organisms were prepared using the colony suspension method [29]. Colonies picked from 24 h old cultures grown on nutrient agar were used to make suspension of the test organisms in saline solution to give an optical density of approximately 0.1 at 600 nm. The suspension was then diluted 1:100 by transfer of 0.1 ml of the bacterial suspension to 9.9 ml of sterile nutrient broth before use to yield 6×105 CFU ml-1.
Determination of the minimum inhibitory concentrations (MIC): The MIC was determined by the micro well dilution method [30] with some modification. This test was performed in sterile flat bottom micro test plates (Tarsons Products Pvt. Ltd.). 150 µl volume of Mueller Hinton broth (MHB) was dispensed into each well and 20 µl of various concentrations of the compounds was added in decreasing order along with 30 µl of the test organism suspension. The final volume in each well was 200 μl (150 μl Mueller Hinton broth, 30 µl of the test organism suspension and 20 μl compound). Two control wells were maintained for each test batch; sterility control (MHB and DMSO) and organism control (MHB, test organism and DMSO). Plates were then incubated at 37°C for 24 h. Experiments were carried out in duplicate. After incubation, 40 µl of INT (2-(4-Iodo phenyl)-3-(4-nitro phenyl)-5-phenyltetrazolium chloride) solution (0.2 mg ml-1) dissolved in sterile distilled water was added to each well [31]. The plates were incubated for further 30 min and were estimated visually for change in color to pink indicating reduction of the dye due to bacterial growth. The highest dilution (lowest concentration) that remained clear corresponded to the MIC.
Determination of the minimum bactericidal concentration (MBC): MBC was determined from all wells showing no growth as well as from the lowest concentration showing growth in the MIC assay for all the samples. Bacterial cells from the MIC test plate were sub cultured on freshly prepared solid nutrient agar plates by making streaks on the surface of the agar. The plates were incubated at 37°C for 24 h overnight. Plates that did not show growth were considered to be the MBC for the compounds used [32]. The experiment was carried out in duplicate.
Results and Discussion
2, 4-disubstituted pyrimidine derivatives (BKD and KDB series):
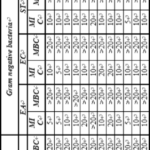
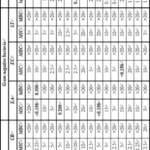
The MIC and MBC values of BKD compounds are presented in Table 5. The compounds exhibited concentration dependent inhibition of growth. All the compounds showed varied levels of MIC and MBC values against studied microorganism. In sterility control (MBH and DMSO), DMSO had no inhibitory effect on the tested organisms. For the Gram positive bacterial strains MIC and MBC varied from <0.156 mg ml-1 to >20 mg ml-1 and 0.250 mg ml-1 to >20 mg ml-1 respectively for BKD series whereas for KDB series, MIC and MBC varied from <0.156 mg ml-1 to >20 mg ml-1 and 10 mg ml-1 to >20 mg ml-1 respectively.
Against S. albus, all compounds showed MIC and MBC values >20 mg ml-1. Against S. aureus, compound BKD-4 and BKD-6 showed lowest MIC (<0.156 mg ml-1) whereas maximum is obseved by BKD-7 having value of 10 mg ml-1. The lowest MBC value is 0.250 mg ml-1 for BKD-6 followed by BKD-4 and BKD-9 (having value 10 mg ml-1). BKD-10 and BKD-12 had minimum MIC value of 0.625 mg ml-1 against C. rubrum whereas MBC values were 20 mg ml-1 for all the studied BKD compounds.
For all the selected Gram negative bacterial strains, MBC values are >20 for all the BKD compounds. The MIC values are minimum i.e., 5 mg ml-1 for BKD-1, BKD-2 and KDB-10 against E. aerogenes. For E. coli also, BKD-2 has minimum value of 5 mg ml-1 whereas a value of 5 mg ml-1 is for BKD-10 against S. typhimuriu which is minimum as comparison to other compounds.
For different fungal strains, all the compounds showed varied levels of MIC and MBC values. Against C. albicans, minimum value of MIC is for BKD-1 and maximum is for BKD-12. MBC values were >20 mg ml-1 for all the studied BKD compounds. The lowest MIC values (<0.156 mg ml-1) shown by BKD-1 (against C. glabrara) and BKD-3 and BKD-5 against C. neoformans. The minimum MBC values are 10 mg ml-1 and 5 mg ml-1 respectively for these two fungal strains.
The inhibition depends on solvent, compound structure and strain. In the present study, solvent is same throught so this parameter is not considered. Table 1 shows that different R groups in these compounds. These compounds have same central nucleus but different substitution. These substitutions are aryl ring with different functional groups. Thus, different substitutions affect different strains differently. In BKD series, BKD-4 and BKD-6 contains 3-CF3 and 4-OCH3 respectively. Thus, 3-CF3 and -OCH3 substitutions are more effective against S. aureus. Whereas 4-chloro present in compound BKD-1 is more effective against fungal strain C. glabrara. Against C. neoformans, BKD-3 and BKD-5 compounds are more effective containing 4-fluoro and 3-chloro, 4-fluoro respectively. For Gram negative bacteria, the studied BKD compounds are not very effective. Thus, these selected Gram negative bacteria are most resistant. Among Gram positive bacteria, S. albus is most resistant.
Table 6 shows that all the KDB compounds have >20 mg ml-1 value of MIC against S. albus. However, S. aureus, KDB-4 and KDB-8 showed lowest MIC (<0.156 mg ml-1) and MBC (10 mg ml-1) values. Thus, for this bacteria, 4-CF3 and 3-Cl are most effective. For C. rubrum, MBC values were >20 mg ml-1 for all the studied KDB compounds but MIC values are minimum (2.5 mg ml-1) for KDB-9.
Thus, 2-flouro substitution is most effective for this strain. All the compounds showed varied and moderate MIC values against E. coli, E. aerogenes and S. typhimurium.However, MBC values are >20 for all the compounds. For the fungal strains, MIC and MBC varied from <0.156 mg ml-1 to >20 mg ml-1 and 5 mg ml-1 to >20 mg ml-1 respectively. Minimum MIC is for KDB-9 containing 2-flouro against C. neoformans.
Fused pyrimidine derivatives (TC, KD and K series):
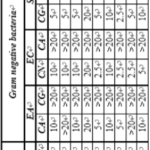
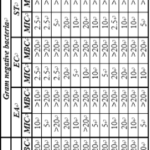
Table 7 shows the MIC and MBC values of TC series. TC-9 showed minimum value of MIC (>0.156 mg ml-1) followed by TC-1 (>0.425 mg ml-1) against S. albus. Whereas TC-5, TC-7, TC-8 and TC-10 compounds are more effective against S. aureu. For C. rubrum, TC-2 and TC-15 showed minimum MIC value of 1.25 mg ml-1. The MBC values are >20 mg ml-1 for all the compounds against S. albus and C. rubrum. Only TC-7, TC-8 and TC-15 had MBC value of 10 mg ml-1.
As shown in Table 2, TC compounds have same central nucleus but different R1, R2 and R3 groups. TC-9 contains 3, 4-di chloro, (CH3)2CH- and 4-OCH3 whereas TC-1 contains 4- chloro, (CH3)2CH- and 4- chloro groups at R1, R2 and R3 positions respectively. Thus, it is observed that when 4- chloro and 4-OCH3 groups are present at R3 MIC is minimum. In TC-2, TC-5, TC-7, TC-8, TC-10 and TC-15 compounds also, 4-chloro and 4-OCH3 groups are present at R3 which causes a decrease in MIC values against S. aureu and C. rubrum.
Against Gram negative bacterial strains, when R1 is 3- chloro, 4-F and 4-OCH3 groups, compounds are more effective against E. aerogenes. TC-13 containing 4-chloro (R1), –CH3 (R2) and 4–Br (R3) shows lowest MIC value against E. coli whereas against S. typhimurium, not a single compound was effective. Against E. coli and S. typhimurium, all the compounds have MBC values >20 mg ml-1. Only TC-2 has lowest MBC of 0.300 mg ml-1 against E. aerogenes.
Table 5: Antibacterial activity data (MIC and MBC in mg ml-1) of BKD series compounds against Gram positive bacteria, Gram negative bacteria and fungal strains.
Table 6: Antimicrobial activity data (MIC and MBC in mg ml-1) of KDB series compounds against Gram positive bacteria, Gram negative bacteria and fungal strains.
Table 7: Antimicrobial activity data (MIC and MBC in mg ml-1) of TC series compounds against Gram positive bacteria, Gram negative bacterial and fungal strains
Table 8: Antimicrobial activity data (MIC and MBC in mg ml-1) of KD series compounds against Gram positive bacteria, Gram negative bacteria and fungal strains.
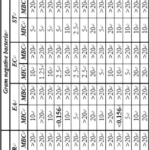
Table 9: Antimicrobial activity data (MIC and MBC in mg ml-1) of KD series compounds against Gram positive bacteria, Gram negative bacteria and fungal strains.
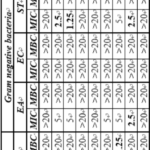
Table 10: Antimicrobial activity data (MIC and MBC in mg ml-1) of TP series compounds against Gram positive bacteria, Gram negative bacteria and fungal strains.
Conclusion
For fungal strains, TC-7 for C. albicans , TC-4 and TC-11-CF3 (R1) and – chloro (R3) groups are present at 4- position in compound TC-7 against C. glabrata and TC-7 and TC-15 for C. neoformans have minimum MIC values. Comparison of different groups at R1, R2 and R3 positions shows that when 4-F group is at R1 position as in TC-4 and TC-15, it is more effective.
This is followed by 4-CF3 ( as in TC-7) and 4- chloro (as in TC-11) at R1 position. In all these four effective compounds, R3 is 4- chloro or 4-OCH3. The R2 position is found to be not very effective. However, all these TC compounds have >20 mg ml-1 MBC values.
Table 8 shows MIC and MBC values of KD series against different bacterial and fungal strains. For this series also, MBC values are not very significant against all the bacterial strains. However, among the three fungal strains, against C. glabrata, KD-7 to KD-12 compounds has MBC value of 10 mg ml-1. For other two strains, values are >20 mg ml-1 for all the compounds.
KD-4, KD-6, KD-9, KD-11 and KD-12 compounds show lowest MIC values against Gram positive bacterial strains. For fungal strains, compounds KD-3, KD-6, KD-7 and KD-12 are most effective. However, all the compounds had little effect against Gram negative bacterial strains.
Table 2 shows the general structure of these derivatives along with different R groups. KD-4 and KD-9 containing 4-F and 3-OCH3 groups, affect S. albus. Against S. aureus, KD-6, KD-11 and KD-12 containing 3- chloro, -4CH3 and 3, 4, 5-tri OCH3 groups are found to be most effective.
Against Gram negative bacteria, overall methoxy and chloro groups at different positions are found to be a little bit effective.
Against fungal strains, 4- chloro and 3-Br containing compounds KD-3 and KD-7 are effective against C. albicans. However, C. glabrata is most inhibited by compound KD-6, KD-7 and KD-12 containing 3-Cl, 3-Br and 3,4,5-tri OCH3 respectively. Against C. neoformans, some compounds show minimum value of MIC to be 2.5 mg ml-1. Thus, for KD series, the selected Gram negative bacteraia are most resistant. Among the studied Gram positive bacteria and fungal strains, C. rubrum and C. neoformans are most resistant for this series.
Table 9 shows the antimicrobial activity data of tetrazolopyrimidine derivatives (K-1 to K-14) against bacterial as well as fungal strains. It is evident from Table 4.5 that against S. albus, MIC value is 5 mg ml-1 for K-13 which is lowest as comparison to other compounds.
Table 2 shows the different substitutions in these compounds. Thus, different substitution affect different strain differently. So, 2-OCH3 group present in K-13, is most effective MIC values are minimum for K-2 and K-6 against S. aureus. Both these compounds again contain methoxy groups at different positions. Thus, methoxy group is most effective against S. aureus also. For C. rubrum, 2.5 mg ml-1 MIC is found to for few compounds; K-9, K-12 and K-14. Thus, for this bacterial strain, 3-Br, 2, 5-di OCH3 and 2-Cl groups are found to be most effective.
Against different Gram negative bacterial strains, K-4 and K-11 compounds are more effective against E. aerogenes. Other compounds had no signifacnt effect against E. coli and S. typhimurium. Thus, 4-F and 3-OCH3 are most effective against E. aerogenes.
Against fungal strains, compounds, only K-3 and K-11 compounds exhibited minimum MIC value of <0.156 against C. neoformans and C. glabrata respectively. K-3 also has lowest MIC values (0.310 mg ml-1) against C. albicans. Thus, 4-chloro ( as in K-3) and 3-OCH3 (as in K-11) groups are most effective against C. neoformans and C. glabrata respectively.
Table 10 shows the MIC and MBC values of imidazothiazole derivatives (TP-1 to TP-9) against nine bacterial and fungal strains. It is observed that among the three Gram positive bacteria, TP-2 and TP-3 against S. albus and TP-7 and TP-8 compounds (2.5 mg ml-1) against S. aureus showed lowest MIC as compared to other compounds. Against C. rubrum, TP-7 has minimum MIC of 1.25 mg ml-1. The general structure of these compounds of TP series along with different substitutions (R) are given in Fig. 4.5. Thus, 4-chloro and 4-bromo are most effective against S. albus, 2,4-di chloro and 4-NO2 against S. aureus and 2,4-di chloro against C. rubrum gives better results. In case of Gram negative bacteria, not a single compound gave significant MIC against E. coli. For Whereas TP-7 compound also exhibited better results for two fungal strains. The MBC values are >20 mg ml-1 for all the compounds against S. albus and S. aureus. However, TP-7 having 2, 4-dichloro substitution, exhibited lowest MBC against C. rubrum.
In Gram negative bacterial strains, TP-8 having 4-NO2 shows the lowest MIC value (2.5 mg ml-1) against E. aerogenes and S. typhimurium. For S. typhimurium, TP-2 and TP-3 also show lowest MIC values (2.5 and 1.25 mg ml-1). Thus, again 4-Chloro and 4-bromo groups are most effective. For E. coli, not a single compound has minimum MIC value. Further, MBC values for all TP compounds are >20 mg ml-1.
Against fungal strains, onlt TP-7 containing 2,4-dichloro groups exhibited better results of MIC and MBC against C. albicans and C. glabrata fungal strains. Other compounds have >20 mg ml-1 values for both MIC and MBC against these strains. For C. neoformans, these TP series compounds are not effective.
Thus, among the studied bacterial and fungal strains, E. coli and C. neoformans are most resistant for this series.
Thus, it is concluded that some of the studied compounds in different series can be used as a lead molecule for further biological study, since these compounds exhibited better activity against different strains.
Conflict of interest
None
Acknowledgments
None
References
1. Anderson I, Terwisscha S A C, Valegard K et al. Towards new β-lactam antibiotics. Cell Mol Life Sciences. 2001; 58: 1897-1906.
2. Geng P F, Liu X Q, Zhao T Q, Wang C C, Liu H M, Zhang J, Wei H M, Hu B, Ma L Y, Liu H M et al. Design, synthesis and in vitro biological evaluation of novel [1,2,3] triazolo [4,5-d] pyrimidine derivatives containing a thiosemicarbazide moiety. Eur J Med Chemistry. 2018; 146: 147-156.
3. Lee H J, Pham P C, Hyun S Y, Baek B, Kim B, Kim Y, Min H Y, Lee J, Lee H Y et al. Development of a 4-aminopyrazolo[3,4-d] pyrimidine-based dual IGF1R/Src inhibitor as a novel anticancer agent with minimal toxicity. Mol Cancer. 2018; 17: 50-66
4. Barakat A, Soliman S M, Al-Majid A M, Lotfy G, Ghabbour H A, Fun H K, Yousuf S, Choudhary M I, Wadood A et al. Synthesis and structure investigation of novel pyrimidine-2,4,6-trione derivatives of highly potential biological activity as anti-diabetic agent. Russ J Bioorg Chemistry. 2015; 41:192–200.
5. Vanitha G K, Ramaiah M, Vaidya V P et al. Synthesis of novel antimicrobial agents encompassing naphthofuran, pyrimidine and thiadiazole moieties. J Chem Pharm Research. 2013; 5: 75–79.
6. Fathalla O A, Zeid I F, Haiba M E, Soliman A M, Abd-Elmoez S I, El-Serwy W S et al. Synthesis, antibacterial and anticancer evaluation of some pyrimidine derivatives. World J Chemistry. 2009; 4: 127–132.
7. Sondhi S M, Singh N, Johar M, Kumar A et al. Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg Med Chemistry. 2005; 13: 6158–6166.
8. Gupta J K, Sharma P K, Dudhe R, Mondal S C, Chaudhary A, Verma P K et al. Synthesis and analgesic activity of novel pyrimidine derivatives of coumarin moiety. Acta Pol Pharmaceutica. 2011; 68: 785–793.
9. Keche A P, Hatnapure G D, Tale R H, Rodge A H, Birajdar S S, Kamble V M et al. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: synthesis, anti-inflammatory and antimicrobial evaluation, Bioorg Med Chem Letters. 2012; 22: 3445–3448.
10. McguiganC, Barucki H, Blewett S, Carangio A, Erichsen J T, Andrei G, Snoeck R, De Clercq E, Balzarin J et al. Highly potent and selective inhibition of varicella-zoster virus by bicyclic furopyrimidine nucleosides bearing an aryl side chain. J Med Chemistry. 2000; 43: 4993–4997.
11. Khalifa N M, Abdel-Rahman A A H, Abd-Elmoez S I, Fathalla O A, Abd El-Gwaad A A et al. A convenient synthesis of some new fused pyridine and pyrimidine derivatives of antimicrobial profiles. Res Chem Intermediates. 2013; 41: 2295-2305.
12. Wardakhan W W, Abdel-Salam O M E, Elmegeed G A et al. Screening for antidepressant, sedative and analgesic activities of novel fused thiophene derivatives. Acta Pharmacutica. 2008; 58: 1–14.
13. Hafez H N, Al-duaij O K, El-gazzar A B A et al. Design, synthesis and pharmacological evaluation of new nonsteroidal anti-inflammatory derived from 3-aminobenzothieno[2,3-d] pyrimidines. Inter J Org Chemistry. 2013; 3: 110–118.
14. Chaykovsky M, Lin M, Rosowsky A, Modest E J et al. 2, 4-diaminothieno [2, 3-d] pyrimidines as antifolates and antimalarials. 2. Synthesis of 2, 4-diamino pyrido [4’, 3′:4,5] thieno [2, 3-d] pyrimidines and 2, 4-diamino-8H- thiopyrano [4′,3′:4,5] thieno [2,3-d] pyrimidines. J Med Chemistry. 1973; 16: 188–190.
15. Panahi F, Yousefi R, Mehraban M H, Nezhad A K et al. Synthesis of new pyrimidine-fused derivatives as potent and selective antidiabetic α-glucosidase inhibitors. Carbohydr Research. 2013; 380: 81–91.
16. Huang B, Li C, Chen W, Liu T, Yu M, Fu L, Sun Y, Liu H, De Clercq E, Pannecouque C, Balzarini J, Zhan P, Liu X et al. Fused heterocycles bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 3: Optimization of [1,2,4] triazolo [1,5-a] pyrimidine core via structure-based and physicochemical property-driven approaches. Eur J Med Chemistry. 2015; 92: 754-765.
17. Abdel Reheim M A M, Baker S M et al. Synthesis, characterization and in vitro antimicrobial activity of novel fused pyrazolo [3,4-c] pyridazine, pyrazolo[3,4-d] pyrimidine, thieno [3,2-c] pyrazole and pyrazolo [3′,4′:4,5] thieno [2,3-d] pyrimidine derivatives. Chem Cent Journal. 2017; 11:112-125.
18. Li Q, Chen Y M, Hu Y G, Luo X, Ko J K S, Cheung C W et al. Synthesis and biological activity of fused furo [2,3-d] pyrimidinone derivatives as analgesic and antitumor agents. Res Chem Intermediates. 2016; 42: 939–949.
19. Bhalgat C M, Ali M I, Ramesh B, Ramu G et al. Novel pyrimidine and its triazole fused derivatives: Synthesis and investigation of antioxidant and anti-inflammatory activity. Arab J Chemistry. 2014; 7: 986-993.
20. Patel T S, Vanparia S F, Gandhi S A, Patel U H, Dixit R B, Chudasama C J, Dixit B C et al. Novel stereoselective 2,3-disubstituted quinazoline-4(3H)-one derivatives derived from glycine as a potent antimalarial lead. New J Chemistry. 2015; 39: 8638-8649.
21. Guzeldemirci N U, Kucukbasmaci O. et al. Synthesis and antimicrobial activity evaluation of new 1,2,4-triazoles and 1,3,4-thiadiazoles bearing imidazo [2,1-b]thiazole moiety. Eur J Med Chemistry. 2010; 45: 63–68.
22. Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M et al. Synthesis and antitubercular activity of imidazo [2,1-b] thiazoles. Eur J Med Chemistry. 2001; 36: 743–746.
23. Dangi R R, Hussain N, Talesara G L et al. Synthesis characterization and biological evaluation of some alkoxyphthalimide derivatives of 3-(4-substituted phenyl)-6,6-diphenyl-3,3a-dihydro-2Himidazo [2,1-b]pyrazolo[3,4-d][1,3]thiazol-7(6H)-one. Med Chem Research. 2011; 20: 1490–1499.
24. Cole D C, Stock J R, Lennox W J, Bernotas R C, Ellingboe J W, Boikess S, Coupet J, Smith D L, Leung L, Zhang G M, Feng X, Kelly M F, Galante R, Huang P, Dawson L A, Marquis K, Lipson S R, Beyer C E, Schechter L E et al. Discovery of N1-(6-Chloroimidazo [2,1-b][1,3]-thiazole-5-sulfonyl)tryptamine as a potent, selective, and orally active 5-HT6 receptor agonist. J Med Chemistry. 2007; 50: 5535–5538.
25. Scribner A, Meitz S, Fisher M, Wyvratt M, Leavitt P, Liberator P, Gurnett A, Brown C, Mathew J, Thompson D, Schmatz D, Biftu T et al. Synthesis and biological activity of anticoccidial agents: 5,6-diarylimidazo[2,1-b][1,3]thiazoles. Bioorg Med Chem Letters. 2008; 18: 5263–5267.
26. Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Farruggia G, Stefanelli C, Masotti L, Kunkel M W et al. Synthesis and antitumor activity of guanylhydrazones from 6-(2,4-Dichloro-5-nitrophenyl) imidazo[2,1-b] thiazoles and 6-pyridylimidazo[2,1-b]thiazoles. J Med Chemistry. 2006; 49: 7897–7901.
27. Riddick J A, Bunger W B, Sakano T et al. Organic Solvents-Physical Properties and Methods of Purification, Techniques of Chemistry, New York, 1986.
28. Sharma A, Gupta S, Sarethy I P, Dang S, Gabrani R et al. Green tea extract: Possible mechanism and antibacterial activity on skin pathogen. Food Chemistry. 2012; 135: 672-675.
29. Haselmann C, European Committee for Antimicrobial Susceptibility Testing (EUCAST): Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution, Clin Micro Infection. 2003; 9: 1-7.
30. Edziri H, Ammar S, Souad L, Mahjoub M A, Mastouri M, Aouni M, Mighri Z, Verschaeve L et al. In vitro evaluation of antimicrobial and antioxidant activities of some Tunisian vegetables. South Afr J Botany. 2012; 78: 252–256.
31. Frey F M, Meyers R et al. Antibacterial activity of traditional medicinal plants used by Haudenosaunee peoples of New York State. BMC Compl Altern Medicine. 2010; 10: 64-73.
32. Akinyemi K O, Oladapo K O, Okwara C E, Ibe C C, Fasure K A et al. Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Compl Altern Medicine. 2005; 5: 6-12.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/