J Med Discov (2018); 3(2):jmd18010; DOI:10.24262/jmd.3.2.18010; Received March 08th, 2018, Revised April 17th, 2018, Accepted April 29th, 2018, Published May 05th, 2018.
A Comprehensive Review: Toxicity of Nanotechnology in the Food Industry
Parisa Ziarati1,2,*, Faezeh Shirkhan1,2, Mahdieh Mostafidi1,2, Maryam Tamaskani Zahedi1,2
1Nutrition & Food Sciences Research Center, Islamic Azad University Medical Sciences Tehran, Tehran-Iran (IAUMST)
2Young Researchers & Elite Club, Islamic Azad University Medical Sciences Tehran, Tehran-Iran (IAUMST)
* Correspondence: Parisa Ziarati, Nutrition and Food Sciences Research Center, Islamic Azad University Medical Sciences, Tehran, Tehran-Iran (IAUMST) . No 99, Yakhchal, Gholhak, Dr. Shariati, Tehran-Iran, 1941933111, Tel: 982122640051, fax: 982122633986. Email: ziarati.p@iaups.ac.ir.
Abstract
Nanotechnology covers many different aspects, such as disease treatment, food security, new materials for identifying pathogens, packaging materials. The best utilize of nanotechnology is reported in agriculture and food. The development of new Nano technology is a new and emerging feature that has been described as having beneficial effects of nanotechnology, but little attention has been paid to Nano-toxicology, and today it is necessary to pay attention to its effects on the biological field. The potential consequences of exposure to Nano-composite foods are important issues and also the risk assessment of Nano-particles should be concerned firmly. In this paper, it has been tried to express nanotechnology in the food chain from production to processing, assessing nanotechnology risk in food and food toxicity and side effects of Nano- particles. Public acceptance of food and food-related products containing Nano-materials will depend on their safety. Consequently, a uniform international regulatory framework for nanotechnology in food is necessary.
Keywords: nanoparticles, food safety, toxicity, nano-science, risk assessment
Introduction
Nanotechnology has captured its capabilities and features in a broad range of applications and industries, and many applications have attracted the attention of many chemical, biological and industrial companies. The novel properties in engineered nanoparticles are widely used and consumer’s products containing engineered nano-materials are quickly rising in food industries.
The food industry is one of the prime industries and a major target of application of nano materials. Food processing refers to the practice adopted by the food and beverages industries to convert the raw plant and animal materials in to ready to serve or eat form of food for consumers. Nano-materials play an effective role in food preservation, packaging process and packaging material. Candies, sweets, and chewing gums have gained popularity because of the use of Nano-materials. This success is based on the sensitivity and efficiency of the products of nano-science. Uses of nano-scale materials in the food industry are likely to improve the processed food. Nano-materials are being used as coated materials in treatment and as diagnostic tools [1].
Substances may enter the body via oral ingestion, inhalation, dermal penetration and intravascular injection and subsequently distribute to any organ system. The advantages and disadvantages of each of the routes are summarized in figure 2 [3].
Toxicology is an important science in which the impact of nanotechnology on living organisms has been considered. Therefore, the main objective of the current study is toxic effects of the nanoparticles.
Applications of Nanotechnology in the food chain
Potential nanotechnologies application areas includes organic and inorganic Nano-additives, food with nanoparticles, Nano-sensors for food quality control and smart packaging, nano-coating and nano-films for kitchenware and foodstuffs, antimicrobial, hygiene coatings, detection of pathogens in food and beverages, self-sanitizing surfaces, polymeric films for food packaging with high antibacterial properties, nano-scale freshness indicators, nano-emulsions for fat reduction, etc. [4].
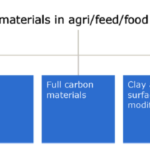
Fig 1. Overview of engineered nanomaterial types applied in agriculture, feed and food sectors[2]
Risk Assessment of Nanotechnology
Although nanotechnology has many benefits and benefits, it is related to food safety and potentially affects not only humans, but animals and the environment.[17]
It is important to note that nano-materials may have toxic effects in the body due to their increased contacting levels which may not be obvious in mass media. In addition, there may be potential and unpredictable risks to use in food packaging
Fig 2. Routes of administration of nanoparticles and their advantages and disadvantages
Though due to lack of regulation and risk knowledge, a few amount of food and food containing nano additives are sold suh as iron in a mixture of nutritional drinks, micelles that contain vitamins; minerals and substances Vegetable in oil carry zinc oxide in cereals.
| Nano-sciences in the food industry | Goals | |
|
Food packaging |
Food packaging[5] | product freshness, flavor stability, longer service life, monitoring, and traceability[5] |
| Nanosensors[6] | determination of microbes, contaminants, pollutants & food freshness [6] | |
|
Food processing |
Food additives[7] | Improve of taste of food[7] |
| Gelating agent[8] | Improve of food texture[8] | |
| Anticaking agent[8] | Improve consistency & prevent lump formation Anticaking agent [8] | |
| Nano-encapsulation[9] | Bioactive delivery, Flavor delivery Nutrient delivery [10] | |
| Nano-emulsions[11] | utilized in the production of food products for sweeteners, flavored oils, salad dressing beverages, as well as other processed foods [12] | |
|
Food improvement |
Food functional[13] | functional food development[14] |
| (Food fortification and modification)[15] | products are targeted into the human system [16] |
Table 1. Application and of nanotechnology in the food industry
Nano rets are also used in plastic bottles. Although consumers will not be exposed to a huge range of foods, but with the emergence of emerging nanotechnology, such as other new technologies, serious questions about the security of an issue require industry attention, as well as policymakers and researchers. It will be ahead. Therefore, the future belongs to new products and processes in the products[12]. Today, there are several possible risks associated with the impact of these products on human health and have raised concerns about the safety, sensitization, toxicity, carcinogenicity, etc. presented in the table 2.
| Concerns about Nano toxicity [18]. |
| Concerns about Nano Immigration to Food Content [19], [20]. |
| Concerned about food products contain insoluble, indigestible, and potentially bio-persistent nano-additives (e.g. metals or metal oxides), or functionalized nano-materials [21]. |
| Concerns about the effect of nanoparticles on the nervous system [22]. |
Table 2. Potential risks or food consumption concerns associated with nanotechnology
Depending on the nature of nanoparticles, have classified them as follows:
- Areas of least concern: where food products contain processed (natural) food nano-structures, which are either digested or solubilized in the gastrointestinal(GI) tract, and are not bio persistent.
- Areas of some concern: where food products contain encapsulated food additives in nano-sized carriers which may not be bio persistent but may carry the encapsulated substances across the GI tract. A greater uptake of food colors or preservatives could take the application outside of the conditions under which the ADI (acceptable daily intake value) was set for the additive.
- Areas of major concern: where food products contain insoluble, indigestible, and potentially bio persistent nano-additives) e.g. metals or metal oxides), or functionalized nano-materials. d. ADME profile (adsorption, distribution, metabolism and elimination) and toxicological properties of which are not fully known at present. Some of the projected applications in the agricultural sector (e.g. nano-pesticides) will also fall in this category. By the addition of nanostructured materials to food, water, and drugs, nanoparticles can be absorbed from the intestine and enter the circulatory system but there is not much research focused on this potential route of entry[24].
Risk Assessment of Nanotechnology in food
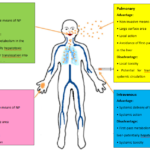
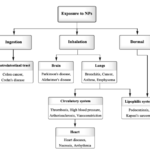
Risk assessment of nano-materials, based on the current approaches to chemical risk assessment framework (hazard identification, hazard characterization, expo-sure assessment and risk characterization) [24]. Figure 3 shows the hypothetical model pathways of NPs uptake in the human body, and target organs where they can accumulate. Nano-particles smaller than 100 nm can easily penetrates skin, and nasal/ pharyngeal mucosa. From there, through the central cardiovascular system, they can reach organs. Toxicity of NPs varies with their properties (size, shape, charge, surface energy, chemical composition and others), and it depends on the properties of living organisms.
Figurer 3. Main entrance pathways and target organs of NPs [23]
Potential wellspring of involuntary nanomaterial contamination in foods encompass the environs, nanomaterials utilization throughout plant and animal product, unintentional release from nano material containing food packaging materials, and residues in foods from nano-materials used as food processing aids or surface coatings on food equipment [17,25]. For example, nano-emulsion and nano -encapsulation technologies are being explored to improve uptake and efficacy of fertilizers, herbicides, and insecticides, which will alter the levels of these substances in foods (that is, could increase or decrease their presence. Environmental contamination of nano-materials resulting from use in a number of other industries is also a potential source of nano-materials in foods, if these materials are present in the environment where food is being produced or in the water being used in food processing. The ability of environmental contamination created by these other industries to potentially affect the food supply has already been illustrated by the presence of many existing industrial contaminants found in foods [26].
The area of food production wherein nanotechnology can have a great impact is in food packaging. Studies have indicated that consumers are more willing to accept the presence of nano-materials in packaging than in. However, the nano-materials in food packaging may potentially migrate to food, which in turn can be ingested or inhaled, or even be transferred through skin contact. Studies on nanoparticles of titanium, silver and CNTs have shown that these materials could enter blood circulation, and their insolubility may cause accumulation in organs. Based on a study by Avella et al. in 2005, only a small amount of particle migration from nano-composites to foods was seen during food packaging. This migration was within the limits prescribed by the European Commission (EC) for silica nanoparticles in clay nano-composites [25] . Similarly, studies on Ag and ZnO by Panea et al. in2013, also showed particle migration to be well below the limits set by the EC. On the other hand, a report shows that nanoparticles of ZnO may potentially lead to genotoxicity in epidermal cells at very less concentration [25-27]. Simon et al. in 2008, reported that decreasing nanoparticle size and polymer viscosity resulted in an increase of the rate of migration in the system[19-26]. In a study by Huang et al. in2011, the temperature at which the packaging was stored and the time were identified as two factors which influenced the amount of nanoparticle migration from the packaging[27-28]. In addition, the use of some nano-composites has raised concerns about environmental contamination, as they may not be bio-degradable. Eco-toxicity studies on such nanoparticles would significantly improve the acceptability of nanotechnology to consumers . Even when some countries permit the release of compounds from packaging, the long term effects of some nano-materials are not yet known. Hence, risk analysis encompasses risk assessment (scientific pursuit), management, communication and the interaction between these integral parts for ensuring the safety and security of public health [27].
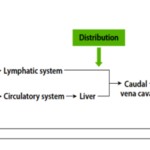
Fig 4. Flow chart describing a nanoparticle’s path through the body from ingestion to excretion
Toxicity of nanoparticles
Nano-toxicology is a sub‐specialty of particle toxicology. It addresses the toxicology of nanoparticles which appear to have toxicity effects that are unusual and not seen with larger particles. The smaller a particle, the greater it’s surface area to volume ratio and the higher its chemical reactivity and biological activity. Because of their large surface area, nanoparticles will, on exposure to tissue and fluids, immediately adsorb onto their surface some of the macromolecules they encounter. This may, for instance, affect the regulatory mechanisms of enzymes and other proteins [28]. Toxicity is one issue that will be uppermost in the minds of the consumer and because nanoparticles are more reactive, more mobile, and likely to be more toxic, this concern must be addressed. There is strong possibility that nanoparticles in the body can result in increased oxidative stress that, in turn, can generate free radicals, leading to DNA mutation, cancer, and possible fatality. It is also not fully understood whether enhancing the bioavailability of certain nutrients or food additives might negatively affect human health [29] .
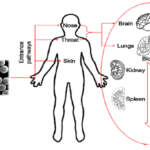
Fig 5. Common exposure routes of nanoparticles in human body are through the skin, respiratory system, and digestive system
Nanoparticle ADME (ADSORPTION, DISTRIBUTION, METABOLISM and EXCRETION) will determine the potential toxicity of the nanoparticle. Absorption refers to the nanoparticle interaction with the gut mucosa and epithelial cells, which ends in the nanoparticle entering into systemic circulation. Distribution or bio-distribution occurs through systemic circulation, during which the particles are distributed to organs such as the liver, kidneys, spleen, heart, lungs, and brain. Metabolism describes the biotransformation of the nanoparticle that occurs through interactions with proteins and lipids in tissues such as the liver and intestinal tissue. The liver, kidneys, and colon are primarily responsible for excretion of nanoparticles and their metabolites [9].
Types of Toxicity in Nanotechnology
Acute toxicity
Acute, sub-acute and chronic toxicity has been investigated for several nano- particles after exposure to rodents (eg copper, selenium, zinc, zinc oxide and titanium dioxide nanoparticles). The results of available oral toxicity studies indicate that, depending on the size, coating and chemical composition of nanoparticle particles, acute toxicity may occur at high doses[30].
Long-term toxicity
So much information about toxicity after acute or chronic exposure to low oral exposure is currently unavailable. Information from the toxicity studies conducted with other ways of exposure indicate that several toxic effects systems in various organ systems may occur
After a long time exposure to nanoparticles, it would affect immune system, inflammation and cardiovascular system. Its effects on the immune system and inflammation may include oxidative stress and / or activation of inflammation of the cytokines in the lungs, liver, heart and brain. Its effects on the cardiovascular system may include the effects and side effects of thrombotic therapy, its effects on cardiac function (acute myocardial infarction and adverb of the effects of heart disease). In addition, cancer and teratogenicity may occur, but no information at this point is still available. The following endpoint is required for the research [31] .
Fig 6. Toxicological effects caused by NPs on the human body [32]
Toxicological effects
The toxicological effect of NPs is thoroughly documented and the major roots of exposure have been identified as dermal, ingestion, and inhalation causing various allied illnesses (Figure 6).
The nanoparticles are likely to be unsafe for the biological system. The research on toxicity of Nano-particles indicates that some of these products may enter the human body and become toxic at the cellular level in the tissues and organs .The materials of these particles may or may not be carcinogenic or allergic but even inert nanoparticles show harmful effects due to some absorbed toxic species or formation of toxic products due to reactions with body fluids. Some nanoparticles may show enhanced catalytic properties to generate highly reactive forms of oxygen that can cause tissue injury including inflammation and other toxic effects [33].
| Type of Nano | Application in foods | Toxic effect | Side effects |
| Nano silver | kitchen supplies (food storage containers, bakeware, cutting boards) Antimicrobial Disinfectants, water purification, kitchen utensils [37] | There is growing evidence that Ag NPs are highly toxic to mammalian cells and human health | Ag-NPs usually generate reactive oxygen species resulting in increased pro-inflammatory reactions and oxidative stress via intracellular signaling pathways. Inflammatory, oxidative, genotoxic, and cytotoxic consequences are associated with silver particulate exposure, and are inherently linked[1] Growing amounts of evidence suggest that orally ingested silver nanoparticles can cross the gastrointestinal barrier, be absorbed into the blood |
| Nano
Titanium |
nutritional supplements and food products, TiO2 prevents microbial growth. itanium dioxide has been approved by USFDA60) and is widely used as a white pigment and food coloran, Candies, sweets, and chewing gums have the highest content of titanium[38] | the TiO2 NPs (anatase/rutile mixtures) showed LD50 in rat >2150 mg/kg leading to low acute oral toxicity | TiO2-NPs can cause several adverse effects on mammalian cells such as increase of reactive oxygen species (ROS) production and cytokines levels, reduction of cell viability and proliferation, induction of apoptosis and genotoxicity [39] TiO2 particles could be transported to other tissues and organs after uptake by gastrointestinal tract [39] |
| Nano
Zinc |
antibacterial activity & packaging[4] | he oral toxicity of ZnO NPs in mice revealed that NPs cause lung, liver and kidney damages | high dose of nano-ZnOs (5000 mg/kg) caused toxicity on development, and altered the zinc metabolism and bio-distribution in mice[40] |
| Nano
Silica |
SiO2 produced in a variety of forms (pyrogenic, gel, sol, precipitate). Amorphous SiO2 has been used for many years in food applications, such as for clearing beers and wines, as anticaking agent to maintain flow properties in powder products and to thicken pastes. Nano-silica (E551) is registered as a food additive in the European Union[38] | also given the reported oral toxicity data
|
A few of the numerous reports clearly demonstrate these findings: ROS generated by the silica surface can induce cell membrane damage via lipid peroxidation that may subsequently lead to increased cellular permeability [41] |
Table 3. Nano-toxicity in some Nano- particles [36]
Some nanoparticles have been found to exhibit negative effects on tissues such as inflammation, oxidative stress and signs of early tumor formation. To determine toxicity profiles, the specifics of the nanoparticle size, shape, solubility, reactivity and other physicochemical parameters must be considered as well as the properties of the substance in a nono form. It is likely that toxicological properties vary among particulate nano-materials, thus a risk assessment must be done on a case by case basis. The safety evaluation of most new substances, according to Handy and Shaw (2007), begins with an overview of physical and chemical properties to ensure safe handling and storage of the material during industrial use and responsible, ethical disposal. Following these basic tests, toxicity of the new substance is studied. Acute and chronic tests, oral toxicity, dermal toxicity, skin irritation as well as mutagenicity tests are carried out on cells [34].
Nanostructures in the food sector may not create a direct effect on human health; however, their nanoscale property may cause some unavoidable side effects. Mechanisms of toxicity induced by nano-materials or NPs have been studied intensively]. Nano-toxicity is principally mediated through the overproduction of ROS, resulting in the induction of oxidative stress and thereby resulting in cells failing to maintain normal physiological redox-regulated functions. Thus, nano-toxicity can lead to DNA damage, unregulated cell signaling, change in cell motility, cytotoxicity, apoptosis, and cancer initiation [35].
Discussion
Food is any substance that contains nutritional value and when consumed it is broken by an organism to produce energy and maintain life. Energy at the cellular level is generated in many ways. A healthier food will produce more energy to maintain metabolism. The main secret of the nanotechnology and food industry is that the cellular level of human cells and food components that are nano-scale and micro-scale can easily be combined with nanoparticles that include nanotechnology in all sectors of the food industry, including processing, packaging, safety And security interact. The interaction of nanoparticles and cells led to discussion of some pessimistic approaches to nano-foods. But the vast potential of nanotechnology across the food industry and its benefits in providing nutritional values, quality packaging, intelligent measurements, has made the minds and research involved in safer techniques for the incorporation of nanotechnology in the food industry[4].
Various forms of nano-materials devised, fabricated such as nanoparticles, nano-fibers, nano-membranes, nanotubes, liposomes, nano-films, etc, are used in food and food technology. It is very essential to estimate the over or abuse of these materials to avoid adverse effects. Study related to nano-toxicity is an appropriate approach to find an optimum, beneficial doses of nano-materials to be used and their elimination from the bio system. Even though it is claimed that the amount used of these products is within safe limits but the amount released directly or indirectly in the environment is comparatively much higher. Perspective of use of nano-materials lies in the hands of personals involved in the field of nanotechnology, food technology and those trying to understand the adverse effects. The insight of the mechanism involved in interaction of nano-materials and bio-systems will guide the appropriate and effective manipulation for the good of man-kind and environment [1].
Available information about safety after oral intake of nano-materials is minimal, and absorption, distribution, metabolism, and excretion information that is essential for risk analysis is yet poorly understood. Moreover, although we are beginning to understand how characteristics of nanomaterials such as particle size, shape, surface properties, and dispersion and aggregation state might influence the biological effects, studies on the safety of nano-materials have not reached the stage at which the results can be used to control quality, develop guidelines for nano-materials, and regulate security of nano-materials. Therefore, quantitative analyses of these characteristics of nano-materials are required, along with assessments of the relationships between these characteristics and the effectiveness, tissue distribution, and safety. Such researches are expected to provide the scientific basis for the risk assessments that are necessary for the development and safe use of nano-materials in the food sector [38].
The safety issues derived from NPs routes of entry and their potential bio-distribution are governed by surface area, shape, agglomeration, aggregation solubility and size with protein (opsonisation) interactions within the host. The size fractions in the nano-scale range have greater lung deposition and rapid systemic translocation having various inflammatory, oxidative and cytotoxic effects on experimental animals than larger particles. With these discussed possible potential routes of NPs, nanotechnology research should proceed with caution. The combination of hazard and production should go hand in hand so as to reduce potential acquisition of NPs through the practice of good manufacturing practice (GMP), good laboratory practice (GLP) and International Standards Organization (ISO). Suitable quality control procedures should be part of the process so as to ensure NPs product safety and quality and hence part of the company quality assurance scheme[42].
Conflict of interest
None of the authors have any conflicts of interest associated with this study.
Acknowledgments
Supports from Nutrition and Food Sciences Research Center, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS) is gratefully acknowledged.
References
1. Kumar LY. Role and adverse effects of nanomaterials in food technology. Journal of Toxicology and Health. 2015;2(1):2. doi: http://dx.doi.org/10.7243/2056-3779-2-2.
2. Peters RJ, Bouwmeester H, Gottardo S, Amenta V, Arena M, Brandhoff P et al. Nanomaterials for products and application in agriculture, feed and food. Trends in Food Science & Technology. 2016;54:155-64. doi: https://doi.org/10.1016/j.tifs.2016.06.008.
3. Yildirimer L, Thanh NT, Loizidou M, Seifalian AM. Toxicology and clinical potential of nanoparticles. Nano today. 2011;6(6):585-607. doi: https://doi.org/10.1016/j.nantod.2011.10.001.
4. Trujillo LE, Ávalos R, Granda S, Guerra LS, País-Chanfrau JM. Nanotechnology applications for food and bioprocessing industries. Biology and Medicine. 2016;8(3):1. doi: http://dx.doi.org/10.4172/0974-8369.100028.
5. Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK, Rather IA. Application of nanotechnology in food science: perception and overview. Frontiers in microbiology. 2017;8:1501. doi: https://doi.org/10.3389/fmicb.2017.01501.
6. He X, Hwang HM. Nanotechnology in food science: Functionality, applicability, and safety assessment. journal of food and drug analysis. 2016;24(4):671-81. doi: https://doi.org/10.1016/j.jfda.2016.06.001.
7. Hamad AF, Jong-Hun HA, Kim BC, Rather IA. The Intertwine of Nanotechnology with the Food Industry. Saudi Journal of Biological Sciences. 2018;25(1):27-30. doi: https://doi.org/10.1016/j.sjbs.2017.09.004.
8. Singh H. Nanotechnology applications in functional foods; opportunities and challenges. Preventive nutrition and food science. 2016;21(1):1-8. doi: 10.3746/pnf.2016.21.1.1.
9. Martirosyan A, Schneider YJ. Engineered nanomaterials in food: implications for food safety and consumer health. International journal of environmental research and public health. 2014;11(6):5720-50. doi: doi: 10.3390/ijerph110605720.
10. Chellaram C, Murugaboopathi G, John AA, Sivakumar R, Ganesan S, Krithika S et al. Significance of nanotechnology in food industry. APCBEE procedia. 2014;8:109-13. doi: https://doi.org/10.1016/j.apcbee.2014.03.010.
11. Dimitrijevic M, Karabasil N, Boskovic M, Teodorovic V, Vasilev D, Djordjevic V et al. Safety aspects of nanotechnology applications in food packaging. Procedia Food Science. 2015;5:57-60. doi: https://doi.org/10.1016/j.profoo.2015.09.015.
12. Ravichandran R. Nanotechnology applications in food and food processing: innovative green approaches, opportunities and uncertainties for global market. International Journal of Green Nanotechnology: Physics and Chemistry. 2010;1(2):72-96. doi: https://doi.org/10.1080/19430871003684440.
13. Brohi RD, Wang L, Talpur HS, Wu D, Khan FA, Bhattarai D et al. Toxicity of Nanoparticles on the Reproductive System in Animal Models: A Review. Frontiers in pharmacology. 2017;8:1-22. doi: https://doi.org/10.3389/fphar.2017.00606.
14. Seaton A. Nanotechnology and the occupational physician. Occupational Medicine. 2006;56(5):312-6. doi: https://doi.org/10.1093/occmed/kql053.
15. Singh PK, Jairath G, Ahlawat SS. Nanotechnology: a future tool to improve quality and safety in meat industry. Journal of food science and technology. 2016;53(4):1739-49. doi: https://doi.org/10.1007/s13197-015-2090-y.
16. Hwang M, Lee EJ, Kweon SY, Park MS, Jeong JY, Um JH et al. Risk assessment principle for engineered nanotechnology in food and drug. Toxicological research. 2012;28(2):73-79. doi: 10.5487/TR.2012.28.2.073.
17. Magnuson BA, Jonaitis TS, Card JW. A Brief Review of the Occurrence, Use, and Safety of Food‐Related Nanomaterials. Journal of food science. 2011;76(6):126-133. doi: https://doi.org/10.1111/j.1750-3841.2011.02170.x.
18. Stern ST, McNeil SE. Nanotechnology safety concerns revisited. Toxicological sciences. 2008;101(1):4-21. doi: https://doi.org/10.1093/toxsci/kfm169.
19. Hannon JC, Kerry J, Cruz-Romero M, Morris M, Cummins E. Advances and challenges for the use of engineered nanoparticles in food contact materials. Trends in Food Science & Technology. 2015;43(1):43-62. doi: https://doi.org/10.1016/j.tifs.2015.01.008.
20. Störmer A, Bott J, Kemmer D, Franz R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends in Food Science & Technology. 2017;63:39-50. doi: https://doi.org/10.1016/j.tifs.2017.01.011.
21. Chaudhry Q, Castle L. Food applications of nanotechnologies: An overview of opportunities and challenges for developing countries. Trends in Food Science & Technology. 2011;22(11):595-603. doi: https://doi.org/10.1016/j.tifs.2011.01.001.
22. Borel T, Sabliov CM. Nanodelivery of bioactive components for food applications: types of delivery systems, properties, and their effect on ADME profiles and toxicity of nanoparticles. Annual review of food science and technology. 2014;5:197-213. doi: https://doi.org/10.1146/annurev-food-030713-092354.
23. Sportelli MC, Picca RA, Cioffi N. Recent advances in the synthesis and characterization of nano-antimicrobials. TrAC Trends in Analytical Chemistry. 2016;84:131-8. doi: https://doi.org/10.1016/j.trac.2016.05.002.
24. Bouwmeester H, Dekkers S, Noordam MY, Hagens WI, Bulder AS, De Heer C et al. Review of health safety aspects of nanotechnologies in food production. Regulatory toxicology and pharmacology. 2009;53(1):52-62. doi: https://doi.org/10.1016/j.yrtph.2008.10.008.
25. Sung JH, Ji JH, Park JD, Yoon JU, Kim DS, Jeon KS et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicological sciences. 2009;108(2):452-61. doi: https://doi.org/10.1093/toxsci/kfn246.
26. Pathakoti K, Manubolu M, Hwang HM. Nanostructures: Current uses and future applications in food science. journal of food and drug analysis. 2017;25(2):245-53. doi: https://doi.org/10.1016/j.jfda.2017.02.004.
27. Ramachandraiah K, Han SG, Chin KB. Nanotechnology in meat processing and packaging: potential applications—A review. Asian-Australasian journal of animal sciences. 2015;28(2):290-302. doi: 10.5713/ajas.14.0607.
28. Ramakrishna D, Rao P. Nanoparticles: Is Toxicity a Concern?. EJIFCC. 2011; 22(4): 92-101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4975312/
29. Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E. Nanotechnologies in the food industry–Recent developments, risks and regulation. Trends in Food Science & Technology. 2012;24(1):30-46. doi: https://doi.org/10.1016/j.tifs.2011.10.006.
30. Gaillet S, Rouanet JM. Silver nanoparticles: their potential toxic effects after oral exposure and underlying mechanisms–A review. Food and Chemical Toxicology. 2015;77:58-63. doi: https://doi.org/10.1016/j.fct.2014.12.019.
31. Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Critical reviews in toxicology. 2010;40(4):328-46. doi: https://doi.org/10.3109/10408440903453074.
32. Ariyarathna IR, Rajakaruna RM, Karunaratne DN. The rise of inorganic nanomaterial implementation in food applications. Food Control. 2017;77:251-9. doi: https://doi.org/10.1016/j.foodcont.2017.02.016.
33. Vishwakarma V, Samal SS, Manoharan N. Safety and risk associated with nanoparticles-a review. Journal of minerals and materials characterization and engineering. 2010;9(5):455-59. https://file.scirp.org/pdf/JMMCE20100500003_31446970.pdf.
34. Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicology letters. 2007;168(2):176-85. doi: https://doi.org/10.1016/j.toxlet.2006.12.001.
35. Iavicoli I, Leso VE, Fontana LU, Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur Rev Med Pharmacol Sci. 2011;15(5):481-508. file:///C:/Users/ASUS/Downloads/Documents/937.pdf.
36. Sirelkhatim A, Mahmud S, Seeni A, Kaus NH, Ann LC, Bakhori SK et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Letters. 2015;7(3):219-42. doi: https://doi.org/10.1007/s40820-015-0040-x.
37. Chellaram C, Murugaboopathi G, John AA, Sivakumar R, Ganesan S, Krithika S et al. Significance of nanotechnology in food industry. APCBEE procedia. 2014;8:109-13. doi: https://doi.org/10.1016/j.apcbee.2014.03.010.
38. Higashisaka K, Yoshioka Y, Tsutsumi Y. Applications and Safety of Nanomaterials Used in the Food Industry. Food Safety. 2015; 3(2):39-47. doi: https://doi.org/10.14252/foodsafetyfscj.2015005.
39. Handy RD, Shaw BJ. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health, Risk & Society. 2007;9(2):125-44. doi: https://doi.org/10.1080/13698570701306807.
40. Wang C, Lu J, Zhou L, Li J, Xu J, Li W et al. Effects of long-Term exposure to Zinc oxide nanoparticles on development, Zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PloS one. 2016;11(10):e0164434. doi: https://doi.org/10.1371/journal.pone.0164434.
41. Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: another variable entity. Particle and fibre toxicology. 2010;7(1):7-39. doi: https://doi.org/10.1186/1743-8977-7-39.
42. Yah CS, Iyuke SE, Simate GS. A review of nanoparticles toxicity and their routes of exposures. Iranian Journal of Pharmaceutical Sciences. 2012;8(1):299-314. doi: http://ijps.ir/article_2193_0.html.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/