J Med Discov (2017); 2(4):jmd17041; DOI:10.24262/jmd.2.4.17041; Received September 15th, 2017, Revised September 31st, 2017, Accepted October 2nd, 2017, Published October 3rd, 2017
Impacts of Oral Reconstruction on Patients with Tongue Squamous Cell Carcinoma
Xing Zhang1, 2, #, Tianrun Liu3, #, Fengjiao Li1, 5, Huan Li1, 4, Liping Wang6,Shuwei Chen1, 2, Shimin Zhuang3, Xidi Wang1, 2, Zhipeng Chen1, 2, Ming Song1, 2, *
1State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, P. R. China.
2Department of Head and Neck Surgery, Sun Yat-sen University Cancer Center, Guangzhou, P. R. China.
3Department of Otolaryngology-Head & Neck Surgery, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, P.R. China.
4Department of Intensive Care, Sun Yat-sen University Cancer Center, Guangzhou, P. R. China.
5Department of Operating room, Sun Yat-sen University Cancer Center, Guangzhou, P. R. China.
6The People’s Hospital of Bao’an District Shenzhen, Shenzhen, P.R. China.
*Corresponding author: Ming Song, 651 Dongfeng Dong Road, Guangzhou 510060, People’s Republic of China. Phone: 86-20-87343300; Fax: 86-20-87343303;
E-mail: songming@sysucc.org.cn.
#These authors contributed equally to this work.
Abstract
Background: The application of reconstruction techniques in head and neck surgery has become the “workhorse” of head and neck surgeons; however, whether reconstruction is appropriate for all head and neck cancer patients remains as a critical issue. To address this question, this study analyzed the impacts of surgical treatment with immediate reconstruction and postoperative radiotherapy on the survival and functional outcomes of patients with different stages of tongue squamous cell carcinoma (TSCC).
Materials and methods: We collected the clinical data of 184 patients with different stages of TSCC. Sixty-eight patients were immediately reconstructed with a variety of flaps. Thirty-three patients underwent postoperative radiotherapy. The recurrence rate, survival time and functional outcomes, such as speech intelligibility and swallowing capacity, were evaluated and compared.
Results: Reconstruction contributed to a reduced recurrence rate and improved overall survival in the patients with T3/T4 TSCC; but did not significantly affect the recurrence rateor overall survival in T1/T2 TSCC patients. Reconstruction is profitable for postoperative speech intelligibility and swallowing capacity in the patients with T3/T4 TSCC, but not significantly in the patients with T1/T2 TSCC.
Conclusions: Surgical resection with immediate reconstruction resulted in improved oncological outcomes and oral function in the patients with T3/T4 TSCC.
Keywords: tongue squamous cell carcinoma, reconstruction,recurrence rate,overall survival, function.
Introduction
Squamous cell carcinoma consists of more than 95% of all oral malignant neoplasm, and tongue squamous cell carcinoma (TSCC) is one of the most common types of oral malignant neoplasm [1, 2]. The clustered association of TSCC with relatively old men is gradually being replaced by an association with clusters of young men [3].Surgical resection remains the primary modality of treatment for the patients with TSCC. Although the 5-year survival rate has been slightly improved with the introduction of new treatments, such as concurrent chemo-radiotherapy, monoclonal antibody targeting epidermal growth factor receptor (EGFR) combined with chemotherapy or radiotherapy [4, 5], TSCC is still associated with poor prognosis due to the high incidence of local and regional recurrence [6, 7].
Extensive resection is critical to achieve a safe surgical margin for patients with advanced TSCC, and it is likely to cause serious functional and cosmetic deficits. Since reconstructive surgery emerged, several studies have demonstrated the reliability and promising outcomes for head and neck defects reconstructed with a variety of flaps [8-10].However, reconstruction is a complex surgical procedure because of the high risk of vascular crisis and donor site morbidity. Additionally, whether reconstruction contributes to improved life quality and overall survival rate in the patients with TSCCremains as a controversial issue [11, 12], and very few researchers have studied on this topic. For this reason, the aim of this study was to analyze more recent data on disease control, survival and functional outcomes in patients with TSCC treated with reconstruction, to make clear the impacts of immediate reconstruction using a variety of flaps in patients with different T stages of TSCC, and to identify which groups of TSCC patients are suitable for reconstruction.
| Variable | No. of Patients (%) |
| Age (years) | |
| ≤ 53 | 94 (51.1) |
| > 53 | 90 (48.9) |
| Gender | |
| Male | 112 (60.9.1) |
| Female | 72 (39.1) |
| T stage | |
| T1 | 71 (38.6) |
| T2 | 78 (42.4) |
| T3 | 22 (12.0) |
| T4 | 13 (7.1) |
| Lymph node metastasis (N) | |
| N0 | 112 (60.9) |
| N1 | 37 (20.1) |
| N2 | 35 (19.0) |
| Stage | |
| I | 56 (30.4) |
| II | 49 (26.6) |
| III | 38 (20.7) |
| IV | 41 (22.3) |
| Pathological grade | |
| I | 132 (71.7) |
| II | 41 (22.3)
|
| III | 11 (6.0) |
Table 1. Clinicopathological features of the 184 TSCC patients.
| Variable | No. of Patients (%) |
| Neck dissection | 175 (95.1) |
| Ipsilateral RND | 13 (7.4) |
| Ipsilateral MRND | 20 (11.4) |
| Ipsilateral SND | 139 (79.4) |
| Ipsilateral RND and contralateral SND | 1 (0.6) |
| Ipsilateral SND and contralateral SND | 2 (1.1) |
| Flap | 68 (37.0) |
| Anterolateral thigh flap (ALTF) | 46 (67.6) |
| Radial forearm flap (RFF) | 17 (25.0) |
| Pectoralis major myocutaneous flap (PMMF) | 3 (4.4) |
| Rectus abdominis flap (RAF) | 1 (1.5) |
| Latissimus dorsi flap (LDF) | 1 (1.5) |
Abbreviations:RND, radical neck dissection;MRND, modified radical neck dissection;SND,selective neck dissection (usually supraomohyoid neck dissection).
Table 2. Surgical treatment for the 184 TSCC patients
Patients and Methods
Clinical data
Patients with TSCC initially treated between January 1, 2005 and April 13, 2010 were recruited in this review. The patients included in this study met the following criteria: (1) histologically proven, untreated resectable primary TSCC; (2) an absence of confounding variables (second primary tumor, prior history of other cancers). Patients with distant metastasis were excluded. Finally, a total number of 184 patients met the inclusion criteria and were enrolled into this study. Follow-up ended on April 30, 2012.
The patients included 112 males and 72 females with an age range of 20 to 81 years (mean, 52.5 years). The TNM classification and staging were based on the 7th International Union Against Cancer (UICC) staging criteria (2010). The patients’ detailed characteristics are summarized in Table 1. All of the 184 patients underwent surgical resection with or without neck dissection.Sixty-eight of the patients underwent simultaneous reconstruction of intraoral defects and the rest 116 patients did not. Seventeen of the 71 (23.9%) patients with T1 TSCC, 26/78 (33.3%) patients with T2 TSCC, 18/22 (81.8%) patients with T3 TSCC and 7/13 (53.8%) patients with T4 TSCC underwent reconstruction. In this study, 175 patients underwent neck dissection, including ipsilateral neck dissection in 172 patients and bilateral neck dissection in 3 patients.
The standard indications for postoperative irradiation were applied, including one or more of the following pathologic findings (risk factors): T3/T4 stage; N2/N3 stage; pathologic lymph nodes of level IV/V; close margins (< 5 mm); perineural invasion or vascular space invasion. Sixty-four of the 184 patients met the indications for postoperative irradiation. Because of the limited financial resources or some other reasons, only 33 of these 64 patients underwent postoperative radiotherapy. The radiotherapy dosage ranged from 50 Gy to 76 Gy. Twenty-three of the 33 patients were still alive at the last follow-up, and the 23 patients received postoperative radiotherapy with a dose range of 50 Gy to 66 Gy (detailed data are presented in Table 2).
Postoperative evaluation of oral function
All survived patients received a postoperative speech and swallowing evaluation using themethod described by Yanai [9]. In short, speech intelligibilitywas estimated by a speech therapist who graded the patients after a conversation as: 5, no sound errors and speech can be easily understood; 4, speech is occasionally misunderstood; 3, speech is understood only when the context of the text is known to the listener; 2, speech is occasionally understood; and 1, speech is completely unintelligible. Speech intelligibilitywas then classified as good (scores 5–4), acceptable (score 3) or poor (scores 1–2). Swallowing capacity was evaluated using the MTF classification based on the method of food intake (M), the time required for food intake (T) and the consistency of food (F) [13]. The method of food intake (M) was classified and scored as: M5, swallowing is unlimited (5 points); M4, capacity for swallowing anything, but occasional aspiration (4 points); M3, capacity to swallow anything prepared in a suitable form (3 points); M2, capacity to swallow small portions of food, but stomach tube is the main means of ingestion (2 points); and M1, capacity to swallow nothing and stomach tube is the only method of ingestion (1 point). The time required for food intake (T) was assessed according to the average time required to eat a daily meal (irrespective of its nature and consistency) as: T5, food intake time is not significantly different before and after therapy. (5 points); T4, food intake time is prolonged by 0 to 10 min (4 points); T3, food intake time is prolonged by 10 to 20 min (3 points); T2, food intake time is prolonged by 20 to 30 min (2 points); and T1, food intake time is prolonged by more than 30 min or is impossible (1 point). The consistency of food that a patient was able to ingest (F) was classified as: F5, capacity to ingest any food (5 points); F4, capacity to ingest soft, chewable food (4 points); F3, capacity to ingest gruel (3 points); F2, capacity to ingest viscous fluids (2 points); and F1, capacity to ingest only non-viscous fluids (1 point). Finally, the M, T and F scores were added and swallowing capacity was classified as good (scores 13–15), acceptable (scores 9–12) or poor (scores ≤8) according to the sum MTF value.
Statistical Analysis
Statistical analysis was performed using Statistical Program for Social Sciences (SPSS) PASW Statistics for Windows version 18.0 (SPSS Inc., USA). The influence of clinicopathological characteristics and treatment strategy on recurrence and survival rates were assessed using Chi-square test. The Kaplan–Meier method was used to plot survival and disease-free interval curves, and log-rank method was used for comparisons between groups. P values were subject to a global significance at the level of 0.05.
| Variable | Observation | Recurrence | P | Survival | P |
| T stage | 0.001 | 0.018 | |||
| T1 | 71 | 14 | 60 | ||
| T2 | 78 | 37 | 50 | ||
| T3 | 22 | 7 | 16 | ||
| T4 | 13 | 8 | 7 | ||
| Lymph node metastasis (N) | <0.001 | <0.001 | |||
| N0 | 112 | 27 | 97 | ||
| N1 | 37 | 17 | 21 | ||
| N2 | 35 | 22 | 15 | ||
| Pathological stage | <0.001 | <0.001 | |||
| I | 56 | 7 | 51 | ||
| II | 49 | 19 | 39 | ||
| III | 38 | 15 | 24 | ||
| IV | 41 | 25 | 19 | ||
| Grade | 0.017 | 0.002 | |||
| I | 132 | 39 | 105 | ||
| II | 41 | 21 | 22 | ||
| III | 11 | 6 | 6 |
Table 3. Influence of clinicopathological characteristics on recurrence and survival in TSCC.
Results
Relationship between clinicopathological characteristics and prognosis in TSCC.
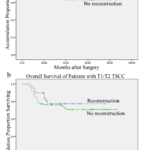
Recurrence was significantly associated with N-stage (P < 0.001), T-stage (P = 0.001), pathological stage (P < 0.001) and tumor grade (P = 0.017) in patients with TSCC. N-stage (P < 0.001), T-stage (P = 0.018), pathological stage (P < 0.001) and tumor grade (P = 0.002) were also significantly associated with overall survival (Table 3). A lower loco-regional recurrence rate (P = 0.015) and higher overall survival rate (P = 0.001) were observed in T3/T4 TSCC patients who received immediate reconstruction, compared to T3/T4 patients who did not undergo immediate reconstruction (Fig 1). However, immediate reconstruction did not significantly affect the recurrence rate (P = 0.513) or overall survival in T1/T2 TSCC patients (P = 0.354; Fig 2), detailed data is presented in Table 4. The recurrence rate (P = 0.397) and overall survival rate (P = 0.150) were not significantly different in TSCC patients who received preoperative chemotherapy and those who did not.
Effect of reconstruction on functional outcomes in stage T1/T2 TSCC patients.
Speech Intelligibility: There was no significant difference inspeech intelligibility between the reconstruction group and the group without reconstruction (P = 0.309; Table 5).
Swallowing Capacity: The surviving patients underwent postoperative swallowing capacity evaluation using the MTF method. None of the surviving patients was stomach tube dependent during the functional survey. There was no significant improvement in patients who received reconstruction, compared to patients who did not undergo reconstruction (P = 0.746; Table 5).
Figure 1. Disease-free interval (1a; P=0.015) and overall survival (1b; P=0.001) of T3/T4 TSCC patients after surgical treatment with (n =17) and without reconstruction (n = 18).
Figure 2. Disease-free interval (2a; P=0.513) and overall survival (2b; P=0.354) of T1/T2 TSCC patients after surgical treatment with (n = 43) and without reconstruction (n = 106).
Effect of reconstruction on functional outcomes in stage T3/T4 TSCC patients.
Speech Intelligibility: Nineteen of the 21 (90.5%) patients in the T3/T4 reconstruction group achieved good speech intelligibility; one patient achieved good speech intelligibility and another achieved acceptable speech intelligibility in the group of T3/T4 patients who did not receive reconstruction. As only two patients from the advanced T3/T4 group without reconstruction survived, we did not pursue further statistical analysis. However, the speech intelligibility outcome of these T3/T4 patients after reconstruction was satisfactory (Table 5).
| Reconstruction | Tumor stage(T1, T2) | Tumor stage (T3, T4) | ||||||||
| Observation
(cases) |
Recurrence
(cases) |
P | Survival | P | Observation
(cases) |
Recurrence
(cases) |
P | Survival | P | |
| Yes | 43 | 13 | 0.513 | 34 | 0.354 | 25 | 7 | 0.015 | 21 | 0.001 |
| No | 106 | 38 | 76 | 10 | 8 | 2 | ||||
Table 4. Influence of reconstruction on recurrence and survival inTSCC patientsat different T stages.
Swallowing Capacity: After reconstruction, 15/21 (71.4%) of the T3/T4 patients acquired good swallowing capacity and 6/21 (28.6%) achieved acceptable swallowing capacity. None of the surviving patients was stomach tube dependent during the functional survey. One surviving patient who did not undergo reconstruction achieved good swallowing capacity and another achieved acceptable swallowing capacity. Due to the small number of surviving patients in the group without reconstruction (two patients), we did not pursue further statistical analysis. However, the swallowing capacity outcome of the T3/T4 patients who underwent reconstruction was satisfactory (Table 5).
| Oral function | Evaluation | Tumor stage (T1, T2) | Tumor stage (T3, T4) | |||
| Flap reconstruction
(cases) |
No flap reconstruction
(cases) |
P | Flap reconstruction
(cases) |
No flap reconstruction
(cases) |
||
| Speech
intelligibility |
Good | 33 | 76 | 0.309 | 19 | 1 |
| Acceptable | 1 | 0 | 2 | 1 | ||
| Poor | 0 | 0 | 0 | 0 | ||
| Swallowing
capacity |
Good | 28 | 66 | 0.746 | 15 | 1 |
| Acceptable | 6 | 10 | 6 | 1 | ||
| Poor | 0 | 0 | 0 | 0 | ||
Table 5. Influence of flap reconstruction on speech intelligibility and swallowing capacityin TSCC patients at different T stages.
Discussion
Reconstruction has been used in the surgical treatment of head and neck cancer for more than five decades, and it has recently become more popular; however, many patients with TSCC still do not achieve the desired results. This study suggests that reconstruction should be applied in selected patients with TSCC.
Controversy of which group of patients with TSCC are appropriate for flap reconstruction always exists. Some clinicians suggest that since the patients with advanced TSCC have a high recurrence rate[14, 15], the benefits of reconstruction are very limited. In contrast, others have advocated that larger defects should be reconstructed when there is a functional or aesthetic loss of structure in the oral cavity after tumor ablation [16-18]. Additionally, immediate reconstruction has been reported to significantly influence the survival of patients with advanced TSCC (T3/T4) [11, 12, 19]. Advanced cancer has always been associated with high recurrence rate, and insufficient surgical margin is the major reason for local recurrence [20, 21]. Extensive resection is necessary in patients with advanced TSCC in order to obtain a safe surgical margin. In this study, we investigated the impacts of reconstruction in patients with different T stage of TSCC, and found that proper reconstruction could enable the head and neck surgeons to perform radical resection of advanced primary tumors (T3/T4).All T3/T4 patients who received reconstruction completed surgical resection (a clinical margin of excision of at least 10 mm). Comparing with T3/T4 TSCC patients who did not receive reconstruction, T3/T4 patients who received reconstruction significantly improved overall survival and lowered rate of recurrence. Probably, because the T1/T2 patients have sufficient surgical margins and sutured directly, in the study, reconstruction did not significantly affect the overall survival and rate of recurrence in the patients with early stageTSCC (T1/T2).
Patients with advancedTSCC have bad functional outcomes after tumor ablation. Malone et al [22]reportedthat patients with stage III and IV squamous cell carcinoma of the tongue base showed worse results of Normalcy of Diet and Understandability of Speech, compared to patients with early-stage cancer.Zelefskyet al. [23]demonstrated that these subjective functional scores deteriorated with increasing T stage in patients with advanced stage oral cavity and oropharyngeal carcinoma. Reconstruction of oral resections rehabilitated the functions in acceptable levels, improving quality of life in these patients [10, 24]. In most literatures, only the postoperative function of patientswho received reconstruction was evaluated. In this study, speech and swallowingfunction of all patients was evaluated, and there was a comparison between patientswho received reconstruction and patients who did not undergo reconstruction. Additionally, postoperative function of patients was analyzed in different T stage. The results suggested that surgical resection combined with reconstruction tended to offer satisfactory speech and swallowing function in advanced patients, while the speech and swallowing functional outcomes were less satisfactory in T3/T4 TSCC patients who did not receive reconstruction. In contrast to the advanced TSCC patients, immediate reconstruction after tumor ablation had no significant effect on speech and swallowing function in early-stage TSCC (T1/T2) patients. Additionally,reconstruction increased the risk of medical complications, including the wound to heal by secondary intention, split-thickness skin or dermal grafting and so on.
The surgical margin is under the direct control of surgeons and has been demonstrated a strong correlation with survival rates [21, 25]. Owing to the advancements of reconstruction, more extensive resections are now available than before. Additionally, T3/T4 TSCC patients who received reconstruction achieve satisfactory speech and swallowing function. Considering the patient’s survival and quality of life, reconstruction is a good choice for patients with advanced TSCC (T3/T4); however,for this circumstance, using a free flap or pedicle flap to reconstruct the oral defect might cause adversities to the patients with early TSCC. So it is still controversial whether to apply a flap reconstruction for the patient with early TSCC, and the decision to perform reconstruction should be made cautiously in patients with early TSCC (T1/T2).
The overall success rate of reconstruction in this study was 97.2%, which is similar to other studies [26, 27]. Flap vascular crisis occurred in six patients due to phlebothrombosis; four of these were successfully salvaged. One patient had total flap necrosis, and as the patient refused repeat reconstruction, the tongue defect was closed directly after the necrotic flap was removed. The other patient had partial flap necrosis. The most commonly observed donor-site morbidity was a broad scar; therefore, to decrease the impact of donor site scarring we suggest the Anterolateral thigh flap (ALTF) is the ideal soft tissue flap for intraoral defect reconstruction, as it has a number of advantages, including enough volume, a flexible shape, secret donor site and long vessel pedicel [10, 28, 29]. Even though many other flaps are available, we usually apply radial forearm flap (RFF) and pectoralis major myocutaneous flap (PMMF) as backup flaps, in case the ALTF fails in vascular crisis.
Compared to patients who did not have the standard risk factors for radiotherapy, the rate of local or regional relapse was significantly higher in patients with one or more risk factor, such as T3/T4 stage, N2/N3 stage, pathologic level IV/V lymph nodes, close margins (< 5 mm), perineural invasion or vascular space invasion. Postoperative radiotherapy is efficient to control local or regional relapse,and it has been previously recommended in patients with one or more risk factors [14, 15, 30-34]. In this study, our results are consistent with previous studies. Postoperative radiotherapy has been reported to negatively influence the functional speech and swallowing outcome in patients with TSCC [17, 35, 36]. While some authors have reported that postoperative radiotherapy at 20 to 40 Gy did not prevent the continued recovery of tongue function with respect to speech, the majority of patients recovered gradually and could still achieve an acceptable functional status and quality of life [9, 23, 37-39]. Schultze-Mosgauet al. [40] reportedthat irradiation at a dose of 40–50 Gy and chemotherapy at a median interval of 1.5 months prior to surgery did not lead to significant histological changes in the recipient vessels. In this study, the impact of postoperative radiation was estimated from two aspect-survival and function. Postoperative radiation improved loco-regional control and overall survival in patients with the risk factors. Speech intelligibility and swallowing capacity were classified as good, acceptable or poor according to the scores. Most patients who underwent postoperative radiation got a low score in the same level, but speech and swallowing capacity was still satisfactory. Postoperative radiation at a dose 50 Gy to 66 Gy partly interfered the life quality for these patients, but the results showed no statistical significance compared to patients who did not undergo postoperative radiotherapy.
In conclusion, it is important to choose the appropriate patients for reconstruction.In this study, immediate oral reconstruction is recommended for advanced TSCC patients. For patients with early TSCC, reconstruction should be reserved and usedselectively, but reconstruction can be considered when there is functional or esthetic loss of the oral cavity. Speech and swallowing functional outcomes are not significantly influenced by postoperative radiotherapy in patients receiving a dose 50 Gy to 66 Gy. A moderate dose of postoperative radiotherapy is recommended for patients with the common/standard risk factors.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This research is supported by the Science and Technology Program Fund of Guangdong Province (No. 2014A020212476).
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E and Forman D. Global cancer statistics. CA Cancer J Clin 2011, 61: 69-90.
- Muir C and Weiland L. Upper aerodigestive tract cancers. CANCER-AM CANCER SOC 1995, 75: 147-153.
- Cooper JS, Porter K, Mallin K, Hoffman HT, Weber RS, Ang KK, Gay EG and Langer CJ. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck 2009, 31: 748-758.
- Lo WL, Kao SY, Chi LY, Wong YK and Chang RC. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. J Oral Maxillofac Surg 2003, 61: 751-758.
- Anaya-Saavedra G, Ramirez-Amador V, Irigoyen-Camacho ME, Zimbron-Romero A and Zepeda-Zepeda MA. Oral and pharyngeal cancer mortality rates in Mexico, 1979-2003. J ORAL PATHOL MED 2008, 37: 11-17.
- Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK, Ho WK and Ho CM. Clinicopathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg 1999, 177: 90-92.
- Rusthoven K, Ballonoff A, Raben D and Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer-AM Cancer Soc 2008, 112: 345-351.
- de Bree R, Rinaldo A, Genden EM, Suarez C, Rodrigo JP, Fagan JJ, Kowalski LP, Ferlito A and Leemans CR. Modern reconstruction techniques for oral and pharyngeal defects after tumor resection. Eur Arch Otorhinolaryngol 2008, 265: 1-9.
- Yanai C, Kikutani T, Adachi M, Thoren H, Suzuki M and Iizuka T. Functional outcome after total and subtotal glossectomy with free flap reconstruction. Head Neck 2008, 30: 909-918.
- Sun G, Lu M, Tang E, Yang X, Wen J and Wang Z. Clinical application of free anterolateral thigh flap in the reconstruction of intraoral defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011, 112: 34-41.
- Mucke T, Wolff KD, Wagenpfeil S, Mitchell DA and Holzle F. Immediate microsurgical reconstruction after tumor ablation predicts survival among patients with head and neck carcinoma. Ann Surg Oncol 2010, 17: 287-295.
- Hanasono MM, Friel MT, Klem C, Hsu PW, Robb GL, Weber RS, Roberts DB and Chang DW. Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck 2009, 31: 1289-1296.
- Fujimoto Y, Matsuura H, Kawabata K, Takahashi K and Tayama N. Assessment of Swallowing Ability Scale for oral and oropharyngeal cancer patients. Nihon Jibiinkoka Gakkai Kaiho 1997, 100: 1401-1407.
- Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ and Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys 1997, 39: 137-148.
- Koo BS, Lim YC, Lee JS and Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. ORAL ONCOL 2006, 42: 789-794.
- Shah N, Saunders MI and Dische S. A pilot study of postoperative CHART and CHARTWEL in head and neck cancer. Clin Oncol (R Coll Radiol) 2000, 12: 392-396.
- Winter SC, Cassell O, Corbridge RJ, Goodacre T and Cox GJ. Quality of life following resection, free flap reconstruction and postoperative external beam radiotherapy for squamous cell carcinoma of the base of tongue. Clin Otolaryngol Allied Sci 2004, 29: 274-278.
- Franceschi D, Gupta R, Spiro RH and Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg 1993, 166: 360-365.
- de Vicente JC, de Villalain L, Torre A and Pena I. Microvascular free tissue transfer for tongue reconstruction after hemiglossectomy: a functional assessment of radial forearm versus anterolateral thigh flap. J Oral Maxillofac Surg 2008, 66: 2270-2275.
- Jerjes W, Upile T, Petrie A, Riskalla A, Hamdoon Z, Vourvachis M, Karavidas K, Jay A, Sandison A, Thomas GJ, Kalavrezos N and Hopper C. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol 2010, 2: 9.
- Binahmed A, Nason RW and Abdoh AA. The clinical significance of the positive surgical margin in oral cancer. ORAL ONCOL 2007, 43: 780-784.
- Malone JP, Stephens JA, Grecula JC, Rhoades CA, Ghaheri BA and Schuller DE. Disease control, survival, and functional outcome after multimodal treatment for advanced-stage tongue base cancer. Head Neck 2004, 26: 561-572.
- Zelefsky MJ, Gaynor J, Kraus D, Strong EW, Shah JP and Harrison LB. Long-term subjective functional outcome of surgery plus postoperative radiotheraphy for advanced stage oral cavity and oropharyngeal carcinoma. AM J SURG 1996, 171: 258-261, 262.
- Archontaki M, Athanasiou A, Stavrianos SD, Korkolis DP, Faratzis G, Papadopoulou F, Kokkalis G and Rapidis AD. Functional results of speech and swallowing after oral microvascular free flap reconstruction. Eur Arch Otorhinolaryngol 2010, 267: 1771-1777.
- Rogers SN, Brown JS, Woolgar JA, Lowe D, Magennis P, Shaw RJ, Sutton D, Errington D and Vaughan D. Survival following primary surgery for oral cancer. Oral Oncol 2009, 45: 201-211.
- Thankappan K, Kuriakose MA, Chatni SS, Sharan R, Trivedi NP, Vijayaraghavan S, Sharma M and Iyer S. Lateral arm free flap for oral tongue reconstruction: an analysis of surgical details, morbidity, and functional and aesthetic outcome. Ann Plast Surg 2011, 66: 261-266.
- Shieh SJ, Chiu HY, Yu JC, Pan SC, Tsai ST and Shen CL. Free anterolateral thigh flap for reconstruction of head and neck defects following cancer ablation. PLAST RECONSTR SURG 2000, 105: 2349-2357, 2358-2360.
- de Vicente JC, Rodriguez-Santamarta T, Rosado P, Pena I and de Villalain L. Survival after free flap reconstruction in patients with advanced oral squamous cell carcinoma. J Oral Maxillofac Surg 2012, 70: 453-459.
- Kimata Y, Uchiyama K, Ebihara S, Nakatsuka T and Harii K. Anatomic variations and technical problems of the anterolateral thigh flap: a report of 74 cases. Plast Reconstr Surg 1998, 102: 1517-1523.
- Majoufre C, Faucher A, Laroche C, De Bonfils C, Siberchicot F, Renaud-Salis JL and Pinsolle J. Supraomohyoid neck dissection in cancer of the oral cavity. AM J SURG 1999, 178: 73-77.
- Zbaren P, Nuyens M, Caversaccio M and Stauffer E. Elective neck dissection for carcinomas of the oral cavity: occult metastases, neck recurrences, and adjuvant treatment of pathologically positive necks. Am J Surg 2006, 191: 756-760.
- Hinerman RW, Mendenhall WM, Morris CG, Amdur RJ, Werning JW and Villaret DB. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head Neck 2004, 26: 984-994.
- Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E and Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys 1996, 36: 999-1004.
- Franceschi D, Gupta R, Spiro RH and Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg 1993, 166: 360-365.
- Shin YS, Koh YW, Kim SH, Jeong JH, Ahn S, Hong HJ and Choi EC. Radiotherapy deteriorates postoperative functional outcome after partial glossectomy with free flap reconstruction. J Oral Maxillofac Surg 2012, 70: 216-220.
- Al-Nawas B, Al-Nawas K, Kunkel M and Grotz KA. Quantifying radioxerostomia: salivary flow rate, examiner’s score, and quality of life questionnaire. STRAHLENTHER ONKOL 2006, 182: 336-341.
- Harrison LB, Zelefsky MJ, Pfister DG, Carper E, Raben A, Kraus DH, Strong EW, Rao A, Thaler H, Polyak T and Portenoy R. Detailed quality of life assessment in patients treated with primary radiotherapy for squamous cell cancer of the base of the tongue. Head Neck 1997, 19: 169-175.
- Hamlet SL, Mathog RH, Patterson RL and Fleming SM. Tongue mobility in speech after partial glossectomy. Head Neck 1990, 12: 210-217.
- Robertson ML, Gleich LL, Barrett WL and Gluckman JL. Base-of-tongue cancer: survival, function, and quality of life after external-beam irradiation and brachytherapy. Laryngoscope 2001, 111: 1362-1365.
- Schultze-Mosgau S, Grabenbauer GG, Wehrhan F, Radespiel-Troger M, Wiltfang J, Sauer R and Rodel F. Histomorphological structural changes of head and neck blood vessels after pre- or postoperative radiotherapy. STRAHLENTHER ONKOL 2002, 178: 299-306.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/