J Med Discov (2017); 2(4):jmd17029; DOI:10.24262/jmd.2.4.17029; Recieved July 4th,2017, Revised July 30th,2017, Accepted August 20th,2017, Published August 26th,2017.
Making of an immuno therapeutic cum immuno prophylactic vaccine against leprosy endowed with multiple properties and useful applications
G.P. Talwar1,*, S.A. Zaheer2, Somesh Gupta3, Dipankar Nandi4, Rohit S Hada1, Jagdish C. Gupta1
1Talwar Research Foundation, New Delhi, India,
2Department of Dermatology, Max Multi Speciality Centre, New Delhi, India,
3Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India,
4Department of Biochemistry, Indian Institute of Science, Bangalore, India
*Correspondence: GP Talwar, Director Research, Talwar Research Foundation, E-8, Neb Valley, Neb Sarai, New Delhi-110068, India. Telephone: +91-011-65022405, E-mail: gptalwar@gmail.com.
Abstract
Reviewed briefly is the development of a unique vaccine against leprosy based on an autoclaved cultivable saprophytic mycobacteria, Mycobacterium indicus pranii (MIP, earlier coded as Mw). MiP is approved by the Drugs Controller General of India and by USFDA. It is transferred to industry and is available to the public.
Besides leprosy, MIP is in use as a potent adjuvant enhancing substantially antibody response to a potential Birth Control vaccine against hCG. MIP has both preventive and therapeutic actions against SP2/O myelomas in mice. It cures marvelously ugly warts on feet and in ano-genital regions by intralesional therapies. It has protective and therapeutic properties against tuberculosis.
Keywords: Mycobacterium indicus pranii, Tuberculosis, Ano-genital warts, Myeloma, Adjuvant
Foreword
In 1970, a WHO team under the leadership of Dr. Howard Goodman visited me at the All India Institute of Medical Sciences to ask me to take up the Headship of WHO Research and Training centre in Immunology for India and South East Asia. I was reluctant. They floored me by stating that India had world’s largest number of lepers. Do we expect Americans to come and solve this problem? I knew nothing about leprosy. I spent the next two summer vacations in Aska (Orissa) and Pogiri (Andhra Pradesh) in a Danish Save the Children Leprosy Home to learn about the disease. Ninety nine percent of humans do not get this disease, the 1% who develop it manifest a spectrum ranging from Tuberculoid (TT) leprosy where the patient has a single lesion with hardly any bacilli to Lepromatous (LL) leprosy where the patient is loaded with M. leprae and has multiple lesions all over the body. Obviously, the immune system of the individual plays a role to manifest this spectrum.
Nature of Immune deficit
Our investigations (Reviewed in 1) indicated that the T cells of the leprosy patient are unable to react to some key antigens of M. leprae to generate signals to the macrophages harbouring the bacilli to kill these and not be a hospitable territory for their growth. This is clearly visible from the results of a study we carried out with monocytes derived macrophages and T cells of both LL and TT leprosy patients. Table 1 summarises the findings.
The permissible multiplication of M. leprae was measured by incorporation of 3H-thymidine. Macrophages do not replicate, hence would not incorporate 3H-thymidine, whereas M. leprae engulfed in macrophages has to synthesise DNA for its multiplication (3). It would be observed that lymphocytes from Tuberculoid(TT) leprosy patients curtail the incorporation of 3H-thymidine, whereas lymphocytes from lepromatous(LL) patients are not competent to do so. In the absence of lymphocytes, M. leprae grows in macrophages derived from both LL or TT patients (2).
Can anything be done to overcome this Immunological Deficit?
Traditionally vaccines have been made to reinforce immunity against infectious micro-organisms. Vaccines are based invariably on killed or attenuated micro-organisms. Such homologous approach was illogical for leprosy, as the very nature of the immunological defect was the inability of their key immune cells to recognize and react to key M. leprae antigens. Hence a heterologous approach was adopted.
We collected 16 strains of cultivable mycobacteria from various sources, included in which were known strains as also atypical mycobacteria. These were coded for investigation. Their ability to cause blast transformation of lymphocytes from both tuberculoid and lepromatous leprosy patients was studied, as well as their ability to generate MIF (macrophage migration inhibitory factor). These studies reported in the entire Golden Jubilee issue of Leprosy in India (4) and elsewhere (5) led to the selection of 5 mycobacterial strains, which were M.vaccae, M.phlei, M.gordonae, ICRC bacillus and Mycobacterium w (Mw).
These strains apparently had the ability to evoke reactions with T cells of not only tuberculoid, but also of lepromatous leprosy patients. A series of investigations were carried out on these 5 shortlisted cultivable mycobacteria, amongst which was their ability to induce lepromin like reaction in not only tuberculoid but also lepromatous leprosy patients. LL patients are lepromin negative and remain lepromin negative even after clearance of bacilli by prolonged treatment with drugs. Lepromin negativity is one of the criteria to classify them as lepromatous. Amongst the many investigations carried out in different parts of the country by recognised Leprologists in leprosy centres, mention may be made of those carried out by Dr. S. Chandhuri at the School of Tropical Medicine Kolkata and Dr.H.K. Kar at the Ram Manohar Lohia Hospital New Delhi. Dr. Chaudhuri immunised 32 leprosy patients with autoclaved Mw. All of them were consistently negative to lepromin before and after extensive treatment with multidrug regimen to become bacillary negative. Twenty of these patients after a single immunisation with Mw became lepromin positive (6). Dr. Kar carried out a field study in Delhi on family members and contacts of leprosy patients. Amongst these were some who were lepromin negative and were likely to develop leprosy, as they were exposed to M. leprae shed by the patient in the environment in which they lived. Sixty seven out of 68 lepromin negative contacts converted to lepromin positivity on immunisation with 2 doses of the vaccine (7). Mycobacterium w fulfilled most of the criteria, which a vaccine for leprosy should have.
| Patient No. | Clinical Status | CPM 3H-thymidine incorporated per 5 × 105 phagocytic cells | |
| Macrophages + Lymphocytes
+ M. leprae |
Macrophages + M. leprae | ||
| 1.
2. 3. |
LL
LL LL |
36,458
53,929 52,354 |
45,628
59,596 83,476 |
| 4.
5. 6. |
TT
TT TT |
6,332
32 381 |
54,969
78,447 26,260 |
Abbreviations: CPM, counts per minute; LL, lepromatous leprosy; TT, tuberculoid leprosy.
After obtaining approval of the Drugs Controller General of India (DCGI) and Ethics committee, autoclaved Mycobacterium w was used as adjunct to standard multidrug regime for treatment of multibacillary leprosy patients. Its inclusion expedited bacterial clearance and shortened the recovery of the patient (8). Upto 68% of LL patients also became lepromin positive on inclusion of the vaccine with drugs.
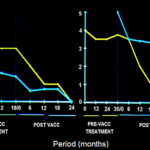
The vaccine was dramatically effective in bacterial clearance of patients recovering rather slowly on treatment with drugs alone. Fig 1 shows that on inclusion of the vaccine, the bacterial index started dropping much faster (9).
Fig.1. Synergistic effect of Mycobacterium w vaccine in slow responders to drugs in two patients. In both, the Bacterial index started declining on immunization with vaccine in addition to the drugs.
Drugs Regulatory Approval
After highly satisfactory Phase III trials in Delhi and Agra followed by field trials in Kanpur Dehat consisting of 272 villages with 420,823 inhabitants, Mycobacterium w vaccine received the approval of the Drugs Controller General of India (DCGI) and was passed on to M/s Cadilla Pharma for manufacture and making the vaccine available to public. The vaccine received also the approval of USFDA. To our knowledge, it is the only vaccine of its type in the world so far.
Molecular Definition of Mycobacterium w
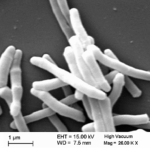
Mw is a cultivable, non-pathogenic mycobacterium. Preliminary gene sequencing of Mw indicated that it is a species distinct from other known mycobacteria (10). A grid of 3 laboratories headed by Prof. S.Hasnain, Prof. Anil Tyagi and Prof. Akhilesh Tyagi carried out the gene sequencing of this mycobacteria. Its genomic constitution and ancestry has been described (11). Being a hitherto undescribed mycobacterium, it has been named as Mycobacterium indicus pranii (MIP) (12). It has been deposited in an International Depository. Fig. 3 is an electron micrograph of this cultivable, non-pathogenic mycobacteria.
Additional properties and uses of MIP
1. Tuberculosis
MIP shares antigens with both M. leprae and M. tuberculosis. Immunization of guinea pigs with MIP prevents their developing tuberculosis on infection with MiP (13). This is the biological efficacy test carried out by M/s Cadilla on each batch of MiP made by the Company for the market. In contrast to BCG, MIP has no genetic restriction for efficacy (13). It is active in both live and killed state, whereas BCG loses efficacy in killed state. MIP has been employed for treatment of Category II , ‘Difficult to treat’ tuberculosis patients with highly encouraging outcomes (13).
2. Potent Adjuvant
It is a potent invigorator of both humoral and cellular immune responses. Its inclusion as adjuvant in a potential Birth Control Vaccine against human chorionic gonadotropin (hCG) enhances antibody titres remarkably (Fig. 4)
Fig. 2. Some representative cases of LL/BL multibacillary patients treated with MDT plus Mw (Mycobacterium indicus pranii)
Fig 3. Electron micrograph of Mw (Mycobacterium indicus pranii)
Fig 4. Antibody titres against human chorionic gonadotropin (hCG). Symbols represent titres in individual mice, Bars give the geometrical mean titres. . Dotted line (----) represents threshold of hCG bioneutralization capacity at 50 ng/ml above which women do not get pregnant (14).
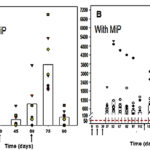
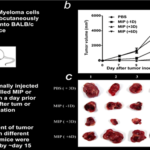
3. Myelomas
It has both preventive and therapeutic action against SP2/O myelomas in mice (Fig. 5).
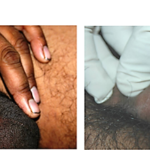
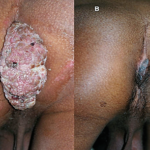
4. Healing of ano-genital warts
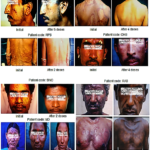
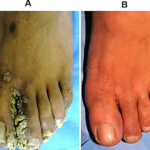
Prof. Somesh Gupta at the All India Institute of Medical Sciences has reported the dramatic action of MIP on healing of ano-genital warts (Fig. 6,7) and on ugly lesions on feet (Fig. 8).
Fig 5. Preventive and therapeutic action against SP2/O myelomas in mice (15).
Fig 6. Healing by MIP of ugly ano-genital warts (A) Patient with giant condylomata (B) Full clearance of lesions with intralesional immunotherapy with MIP (16).
Fig 7. (A) Before treatment (B) After treatment with MIP (13).
Fig 8. Cure by MiP of warts on feet. (A) Before treatment and (B) After 5 months of treatment with MIP (17).
To sum up, Mycobacterium indicus pranii (MIP) is not only a highly effective vaccine against leprosy with bothImmuno-therapeutic and immuno-prophylactic properties, it is a very good, safe adjuvant to enhance antibody titres against hCG. It prevents and cures SP2/O myelomas and has astonishing curative action against feet and ano-genital warts.
Conflict of interest
None
Acknowledgments
The work reviewed in this article received research grants from the Indian Council of Medical Research, Department of Science & Technology and the Department of Biotechnology, Government of India.
References
1. Talwar GP. An immunotherapeutic vaccine for multibacillary leprosy. Int Rev Immunol. 1999; 18: 229-249.
2. Talwar GP, Krishnan AD, Jha P, Mehra V. Intracellular Growth of an obligatory parasite Mycobacterium Leprae. Host Bacterial Interactions. Biochimie. 1974; 56: 231-237.
3. Talwar GP, Krishnan AD, Gupta PD. Quantitative evaluation of the progress of intracellular infection in vitro: DNA synthesis in Mycobacterium leprae in cultivated blood monocytes. Infect Immun.1974; 9, 187-191.
4. Talwar GP and others in the Entire Golden Jubilee Issue of Leprosy in India. Oct. 1978; 50, 492-598.
5. Mustafa AS, Talwar GP. Five cultivable mycobacterial strains giving blast transformation and leukocyte migration inhibition of leukocytes analogous to Mycobacterium leprae. Leprosy in India. 1978; 50:498-508.
6. Chaudhuri S, Fotedar A, Talwar GP. Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium w. Int J Lepr & Other Mycobact Dis. 1983; 51(2):159-68.
7. Kar HK, Sharma AK, Mishra RS, Mukherjee A, Mukherjee R, Beena KR, Kaur H, Nair SK, Talwar GP. Induction of lepromin positivity by a candidate anti-leprosy vaccine Mycobacterium w in lepromin negative healthy contacts of multibacillary leprosy patients. Indian J Lepr. 1992;64 (4):495-500.
8. Zaheer SA, Mukherjee R, Ramkumar B, Mishra RS, Sharma AK, et al. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J Infect Dis. 1993;167(2):401-410.
9. Zaheer S. A, Mukherjee A, Ramesh V et al. Immunotherapy benefits multibacillary patients with persistently high bacteriological index despite long term multidrug therapy. Immunol Infect Dis. 1995; 5: 115-122.
10. Reddi PP, Amin AG, Khandekar PS, Talwar GP. Molecular definition of unique species status of Mycobacterium w, a candidate leprosy vaccine strain. Int J Lepr & Other Mycobact Dis. 1994; 62:229-236.
11. Saini V, Raghuvanshi S, Talwar GP, Ahmed N, Khurana JP, Hasnain SE, Tyagi Akhilesh K, Tyagi Anil K. Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS One. 2009; 4: e6263.
12. Talwar GP, Ahmed N, Saini V. The use of the name Mycobacterium w for the leprosy immunotherapeutic bacillus creates confusion with M. tuberculosis-W (Beijing strain): a suggestion. Infect Genet Evol. 2008; 8: 100-101.
13. Talwar GP, Gupta JC, Mustafa AS, Kar HK, Katoch K, Parida S, Reddi P, Ahmed N, Saini V, Gupta S. Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics: Targets and Therapy. 2017; 11: 1-9.
14. Purswani S, Talwar GP. Development of a highly immunogenic recombinant candidate vaccine against human chorionic gonadotropin. Vaccine. 2011; 29:2341-2348.
15. Rakshit S, Ponnusamy M, Papanna S, Saha B, Ahmed A, Nandi D. Immunotherapeutic efficacy of Mycobacterium indicus pranii in eliciting anti-tumor T cell responses: critical roles of IFNɣ. Int J Cancer. 2012; 130: 865-875.
16. Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: an open label pilot study. J Eur Acad Dermatol Venereol. 2008; 22:1089-1093.
17. Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol. 2014; 80:509-514.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/