J Med Discov (2017); 2(3):jmd17028; DOI:10.24262/jmd.2.3.17028; Recieved July 3rd,2017, Revised August 18th,2017, Accepted August 30th,2017, Published September 8th,2017.
Advanced Molecular Imaging
Yichen Ding, Ph.D.1,2, * Jing Yu, Ph.D.3, Mingzhu Zhang, MD, Ph.D.4
1 Department of Bioengineering, University of California, Los Angeles, CA 90095
2 Department of Biomedical Engineering, College of Engineering, Peking University, Beijing 100871, China
3 College of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou, 310014, China
4 Department of Anesthesiology, Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing 100021, China
* Correspondence: Yichen Ding. ycding@g.ucla.edu
Abstract
Molecular imaging is applied to in vivo characterize biological dynamics at the cellular and molecular level. As an advanced imaging technology in the fundamental study and clinical diagnosis, molecular imaging has significantly changed our understanding of physiological and pathological changes, and it has revolutionized the disease diagnosis and the study of drug delivery. Different imaging modalities and advanced biomedical applications are introduced in this review.
Keywords: Molecular imaging; multi-modality imaging.
Introduction
Imaging is one of the few technologies that can generate longitudinal data sets in intact host environments. Molecular imaging was first proposed in 1999 [1]. The imaging methods and probes are applied to dynamically track the biological processes at the cellular and molecular level for quantitative study. From the perspective of clinical diagnosis, the core of molecular imaging is to quantitatively trace physiological, pathological, metabolic and functional changes for the occurrence of disease by advanced imaging methods. With the advent of various imaging modalities and molecular probes, molecular imaging has been beneficial to medical diagnosis and treatments at the early stage. In this context, molecular imaging has been greatly improved during the past few decades due to various imaging systems and contrast agents.
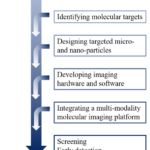
To initiate a molecular imaging study, we need to involve the first step to identify a biochemical process or pathology of interest, and to noninvasively assess the significance of visualizing this process via X-ray computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), fluorescence molecular tomography (FMT), Cherenkov luminescence tomography (CLT), ultrasound imaging (US), photoacoustic tomography (PAT) and other tools of molecular imaging (Figure 1). The second step is to decide on a molecular target that will enable direct or indirect visualization of the phenomena of interest. This is usually related to an appropriate imaging modality and an imaging contrast agent if necessary. Some chemistry and labeling are required to synthesize the contrast agent, that is, both targeting and signaling components are contained in the contrast agent. A number of in vitro (molecular/cell biology- based) and in vivo (animal model-based) tests are required to evaluate the specificity and selectivity of the imaging method for visualizing the phenomena of interest. FDA approval is required if the clinical study is the ultimate goal. In addition, developing a mathematical model and building a three-dimensional reconstruction might be necessary so that meaningful data can be extracted from images [2, 3]. In regard to imaging systems, CT, MRI, PET, SPECT, FMT, CLT, US and PAT are mainly involved in this review.
Computed tomography (CT)
Computed tomography (CT) relies on different X-ray attenuation coefficient of distinct tissue. The first clinical application of CT was reported in 1971 [4], and then researchers upgraded the CT system to different generations to reduce imaging time and improve image
Figure 1. A general scheme of a molecular imaging study.Fig 1: upper endoscopy, white tipped villi
quality in the past decades. The first spiral CT was introduced to achieve continuous scanning, being recognized as the current mainstream of the CT system. In the late 1990s, the multiple-detector CT with superb temporal and axial resolution was developed and was specifically proper for heart imaging [5]. The dual-energy CT was not only dependent on attenuation coefficients but also able to distinguish different compositions of materials by generating X-ray with different energy levels [6]. Generally, the spatial resolution of CT is about 0.5 ~ 1.0 mm for clinical diagnosis, while it is around 0.05 ~ 0.2 mm for animal study. In clinics, due to the high frame rate and the simple apparatus, CT has been mainly used for assessing renal malignancies [7], discriminating between calcium and uric acid renal stones [8], determining bone mineral density [9, 10] and imaging gout [11]. It is more suitable than invasive coronary angiography for locating and characterizing coronary plaques [12] and is also being successfully used to predict coronary events and for long-term stent assessment [13]. However, CT is still limited by the high radiation doses and less contrast for soft tissue imaging. In addition, using iodine as the contrast agent is also adverse to kidney function [14].
Magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) utilizes the electromagnetic wave generated by the nuclei in the magnetic field to reconstruct the architecture of organs. Various information of soft tissues can be extracted by employing different pulse sequences, such as dynamic contrast-enhanced MRI (DCE-MRI), diffusion-weighted MRI (DW-MRI) and blood-oxygen-level dependent MRI (BOLD-MRI). In addition to providing structural information, magnetic resonance spectroscopy (MRS) is also able to determine the molecular structure and composition. This technology can be applied to acquire biochemical spectra of distinct substances, which are used for tracing the composition and capacity change. For instance, choline, lactate and phospholipids were applied as indicators for tumor imaging and neural system disorders [15-17]. In clinics, MRI has been used to study many aspects of brain function and dysfunction, including memory [18], sensory perception [19], cognition [20], and depression [21]. With the aid of targeted MRI agents, some other applications of early atherosclerotic events [22] and glioma imaging [23] were reported as well. In comparison to CT, MRI is more appropriate for soft tissue imaging without exogenous contrast agents. As a non-invasive and radiation-free imaging method, MRI provides soundly spatial resolution as high as 1 mm in the clinical diagnosis. Compared to optical fluorescence imaging, MRI is not limited by penetration depth [24]. However, MRI is less sensitive, and it also requires imaging media and longer acquisition time. Therefore, MRI is not proper for dynamic imaging due to insufficient temporal resolution.
Nuclear medical imaging
Nuclear medical imaging mainly includes positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Both PET and SPECT inherently provide quantifiable information related to physiological and metabolic changes in three dimensions.
The basic principle of PET imaging is based on the decay and annihilation of the radionuclide which is injected into the body as a tracing marker. The advanced detector for PET is critical to the acquisition of high resolution metabolic images. Recently, with the advent of scintillators and optoelectronic components [25, 26], time-of-flight technology (TOF) has been successfully applied to PET systems to provide more accurate information as well as improving the signal-to-noise ratio [27]. Locating the spatial distribution of biomarkers precisely has been feasible since the depth of information (DOI) was able to be well extracted with the development of electronics [28]. A coincidence event is assigned to a line of response (LOR) joining the two relevant detectors. In this way, positional information is gained from the detected radiation due to the electronic collimation instead of the physical collimator. In comparison to physical collimation, the electronic collimation has better sensitivity and uniformity of the response function of the point source. The spatial resolution of clinical PET is about 4 ~ 6 mm, while the resolution is around 2.5 mm for the neural imaging and 1~2 mm for pre-clinical study in small animals [29]. 18F-FDG is widely applied for tumor detection, stratification and prognosis during PET imaging in clinics [30].
SPECT is derived from the Gamma camera, and therefore it is able to be used for mapping the spatial distribution of radionuclide in the organs when 99Tcm, 123I and some other radioactive isotopes are used for tomography or whole-body imaging. The spatial resolution can be enhanced by physically placing a simple pinhole collimator in the SPECT system regardless of the pixel size [31]. Subsequently, another strategy of multi-pinhole with coding algorithm was proposed to improve the spatial resolution as well as ameliorating sensitivity [32, 33]. With the advent of semiconductor and crystal manufacturing, cadmium zinc telluride (CZT) detector which has no conversion between photons and electrons has been employed in the SPECT system [34, 35]. In clinical diagnosis, due to the cost-effective equipment, high specificity and various biomarkers, SPECT has already been widely used for oncology imaging, myocardial perfusion imaging and other diagnosis.
Optical fluorescence imaging
Optical fluorescence imaging mainly includes fluorescence microscopy, fluorescence molecular tomography (FMT) and Cherenkov luminescence tomography (CLT) [36-47]. In regard to fluorescence imaging, spontaneous fluorescence [48] and excited fluorescence [49] are used as in vivo molecular probes for the physiological process in the tissue [50]. With the advent of gene expression, more and more fluorescent proteins of which the spectra range from ultra violet to near-infrared are developed for fluorescence imaging. The main applications of molecular probes include gene and protein expression [51, 52] as well as tumor metastasis [53].
FMT combines fluorescence imaging and reconstruction algorithm together to depict three-dimensional distribution of molecular probes in the body. The first imaging case of gliomas in the nude mouse was reported in 2002 [54], and it revealed the advantages of FMT and demonstrated the potential applications in the drug delivery. Compared to CT, PET and SPECT, FMT provides higher sensitivity and non-radioactive probes for the long-term quantitative study. However, due to the shallow penetration depth of photons, FMT is still limited in clinical applications except for animal study. Recently, a miniature dual-axes NIR confocal endoscopy [55] and a hand-held optical imager [56] have been reported for screening diseases at the early stage.
CLT is based on the Cherenkov effect which was discovered in the early 20th century [57]. It has the potential to be applied in clinical diagnosis since CLT also includes 18F-FDG as the contrast agent. CLT integrates the high sensitive molecular probes used in PET and the cost-effective system employed in FMT for molecular imaging, and therefore CLT is able to concurrently reveal both functional and structural information [58]. The first CLT study in mice was reported in 2009, and the feasibility was verified by comparing with PET results [59].
Ultrasound
The mechanism underlying ultrasound imaging is pulse-echo, that is, the high frequency acoustic wave starts from the ultrasonic transducer and then it is reflected back by the biological tissue. The final two/three-dimensional output is able to be reconstructed in combination with algorithms. The higher the frequency, the better the resolution. However, the imaging depth degrades because of scattering. Ultrasound imaging is subject to speckle effect and low contrast, but it still plays a critical role in clinical diagnosis due to the high temporal resolution and real-time imaging. In addition to diagnosis, ultrasound is also a proper candidate for treatments with the advantage of its mechanistic property. By virtue of non-radioactive micro-bubble agents, the sensitivity of ultrasound was dramatically enhanced for the imaging of microvasculature, angiogenesis and inflammation [60-64]. For instance, moieties targeted toward specific angiogenic markers, including monoclonal antibodies against murine áv-integrins, have been conjugated to micro-bubbles for observation of angiogenesis [61]. Furthermore, endothelial cell adhesion molecules have been attached to micro-bubble shells for visualization of P-selectin, providing insight on molecular aspects of inflammation [62]. In addition, dual-targeted contrast agent was reported for in vivo tumor angiogenesis with better image quality [65]. By conjugating with the specific antibody and peptide on the surface of micro-bubble agents, ultrasound is feasible to reveal the information of physiological and molecular processes in the body.
Photoacoustic tomography (PAT)
Photoacoustic tomography (PAT) is an emerging biomedical imaging modality, integrating optical absorption as image contrast with ultrasound detection together [66]. PAT is based on PA effect, which refers to the generation of sound waves after the item absorbs intensity-varying electromagnetic waves. PA effect is highly sensitive to tissue optical absorption properties. In general, the scattering coefficient for ultrasound in soft tissues is two to three orders less than light. Thus, the soft tissue is nearly “transparent” to ultrasound. By detecting less scattered ultrasound waves, PAT can therefore conserve high resolution imaging of optically absorbing targets in deep tissues. During the past decades, PAT has successfully imaged multi-scale tissues: from sub-molecular organelles to subcutaneous cancer tissues in vivo [67]. PAT has great advantages in imaging subcutaneous diseases, such as skin tumor or burn wound [68]. For PAT, its high sensitivity to blood circulation brings great opportunities in study microcirculation system in shallow or optical clearing tissue. For instance, human finger cuticle has been imaged in vivo with superior contrast [69]. Another very important application is ocular imaging, which has been successfully demonstrated in the animal study [70-72]. With the system becoming more compact and stable, PAT systems has a great potential to be a powerful clinical imaging instrument. From ultraviolet to infrared regions, distinct molecules have different absorption spectra. Thus, any component with enough distinct absorption behaviors with surrounding medium can be imaged by PAT at appropriate illumination wavelengths [73, 74]. In the application of PAT, optical absorbers can be classified into two groups: one is endogenous molecules, and the other is exogenous agents. With the aid of exogenous contrast agents, PAT can now image the tissue that has not enough natural optical absorption contrast with environmental medium, such as the lymphatic vessels [75, 76]. Moreover, due to the enhanced permeability and retention (EPR) effect at the tumor site, various exogenous contrast agents, particularly nanoparticles, have been used for tumor imaging by PAT [77].
Multi-modality imaging
With numerous molecular targets identified for various diseases and advances in material engineering for nano- / micro-particle contrast agents, a large potential exists to expand molecular imaging beyond nuclear medicine approaches. Multi-modality imaging was proposed to concurrently acquire anatomical structure, physiological process and other genetic information while improving the specificity and accuracy of clinical diagnosis. Different imaging methods are able to compensate with each other, and therefore multi-modality imaging system is fundamental to the next generation of molecular imaging. Clinical practice has validated that multi-modality imaging plays a critical role in the diagnosis and treatments at the early stage [78]. Therefore, developing the multi-modality imaging system and the corresponding molecular probe becomes the trend of molecular imaging. The first PET/CT system was reported in 1992 [79], and it was successfully applied in the clinic in 2001. This dual-modality imaging system has been mainly used for tumor diagnosis and prognosis [80]. Later, the first PET/SPECT/CT system for animal study and the PET/MRI for clinical diagnosis were subsequently launched [81, 82]. In 2013, the first quad-modality prototype for molecular imaging was accomplished in China [83]. This system integrated CT, PET, SPECT and FMT for pre-clinical study of physiological and metabolic processes, while it demonstrated superb sensitivity and specificity.
Figure 2. Timeline representation of current and future utilization of molecular imaging.
Conclusion
Imaging has become an indispensable tool in cancer research, clinical trials and medical practice. Currently, molecular imaging systems enable doctors to see where a tumor is located in the body. Sooner or later, it is expected that these systems will also help to visualize the expression and activity of particular molecules, cells and biological processes that influence the behavior of tumors (Figure 2). The translation of imaging technologies and contrast agents into the clinic is the most fundamental issue in the molecular imaging field. It is clear that the quantitative and comprehensive data will be the trend, and therefore quantification and integration are some of the more immediate needs. With the deeper understanding of the molecular basis of disease, the transformative promise of imaging is likely to be fulfilled soon. Applying the new molecular imaging tools will make a fundamental improvement in how cancer is understood in vivo. Furthermore, by using the advanced molecular imaging methods, doctors could allow earlier detection, stratification of patients for treatment, and objectively evaluate new therapies in a given patient. The outcome will be considerably better management and care of those with cancer.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgments
There is no funding supports this work. The authors acknowledge all the colleagues at Peking University for kind discussion.
References
- Weissleder R. Molecular imaging: principles and practice. PMPH-USA; 2010.
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92(2):897-965.
- Xiao J, Mu J, Liu T, Xu H. Dig the root of cancer: targeting cancer stem cells therapy. J Med Discov. 2017;2(2):17003.
- Paxton R, Ambrose J. The EMI scanner. A brief review of the first 650 patients. The British Journal of Radiology. 1974;47(561):530-65.
- Prokop M. General principles of MDCT. Eur J Radiol. 2003;45:S4-S10.
- Di Chiro G, Brooks RA, Kessler RM, Johnston GS, Jones AE, Herdt JR et al. Tissue signatures with dual-energy computed tomography. Radiology. 1979;131(2):521-3.
- Graser A, Becker CR, Staehler M, Clevert DA, Macari M, Arndt N et al. Single-phase dual-energy CT allows for characterization of renal masses as benign or malignant. Invest Radiol. 2010;45(7):399-405.
- Primak AN, Fletcher JG, Vrtiska TJ, Dzyubak OP, Lieske JC, Jackson ME et al. Noninvasive differentiation of uric acid versus non–uric acid kidney stones using dual-energy CT. Acad Radiol. 2007;14(12):1441-7.
- De A, Loening AM, Gambhir SS. An improved bioluminescence resonance energy transfer strategy for imaging intracellular events in single cells and living subjects. Cancer Res. 2007;67(15):7175-83.
- Zhang J, Yan C-H, Chui C-K, Ong SH. Accurate measurement of bone mineral density using clinical CT imaging with single energy beam spectral intensity correction. IEEE T Med Imaging. 2010;29(7):1382-9.
- Johnson TR, Weckbach S, Kellner H, Reiser MF, Becker CR. Clinical image: Dual‐energy computed tomographic molecular imaging of gout. Arthritis Rheum. 2007;56(8):2809-.
- Sun Z, Ng K. Multislice CT angiography in cardiac imaging. Part II: clinical applications in coronary artery disease. Singapore Med J. 2010;51:282-9.
- Nieman K. Noninvasive stent imaging with MSCT. EuroIntervention. 2009;5:D107-11.
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17(5):545-80.
- Boesch C. Musculoskeletal spectroscopy. J Magnet Res Imaging. 2007;25(2):321-38.
- Soares D, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64(1):12-21.
- van der Graaf M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J. 2010;39(4):527-40.
- Ziemus B, Baumann O, Luerding R, Schlosser R, Schuierer G, Bogdahn U et al. Impaired working-memory after cerebellar infarcts paralleled by changes in BOLD signal of a cortico-cerebellar circuit. Neuropsychologia. 2007;45(9):2016-24.
- Azulay H, Striem E, Amedi A. Negative BOLD in sensory cortices during verbal memory: a component in generating internal representations? Brain Topogr. 2009;21(3-4):221-31.
- Fink GR. Functional MR imaging: from the BOLD effect to higher motor cognition. Suppl Clin Neurophysiol. 2004;57:458-68.
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61(2):198-209.
- Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96(3):327-36.
- Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D et al. In vivo MRI detection of gliomas by chlorotoxin‐conjugated superparamagnetic nanoprobes. Small. 2008;4(3):372-9.
- Chatham JC, Blackband SJ. Nuclear magnetic resonance spectroscopy and imaging in animal research. IlAR J. 2001;42(3):189-208.
- Miyaoka RS, Kohlmyer SG, Lewellen TK, editors. Performance characteristics of micro crystal element (MiCE) detectors. Nucl Sci Symp Conf Rec; 2000: IEEE.
- Szczesniak T, Moszynski M, Swiderski L, Nassalski A, Lavoute P, Kapusta M, editors. Fast photomultipliers for TOF PET. Nucl Sci Symp Conf Rec; 2007: IEEE.
- Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med. 2007;48(3):471-80.
- Miyaoka R, Lewellen T, Yu H, McDaniel D. Design of a depth of interaction (DOI) PET detector module. IEEE T Nucl Sci. 1998;45(3):1069-73.
- Tai Y-C, Chatziioannou AF, Yang Y, Silverman RW, Meadors K, Siegel S et al. MicroPET II: design, development and initial performance of an improved microPET scanner for small-animal imaging. Phys Med Biol. 2003;48(11):1519.
- Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580.
- Weber DA, Ivanovic M. Ultra-high-resolution imaging of small animals: implications for preclinical and research studies. J Nucl Cardiol. 1999;6(3):332-44.
- Meikle SR, Kench P, Weisenberger AG, Wojcik R, Smith MF, Majewski S et al. A prototype coded aperture detector for small animal SPECT. IEEE T Nucl Sci. 2002;49(5):2167-71.
- Ochos AV, Ploux L, Mastrippolito R, Charon Y, Laniece P, Pinot L et al. An original emission tomograph for in vivo brain imaging of small animals. IEEE T Nucl Sci. 1997;44(4):1533-7.
- Kastis GA, Wu MC, Balzer SJ, Wilson DW, Furenlid LR, Stevenson G et al., editors. Tomographic small-animal imaging using a high-resolution semiconductor camera. Nucl Sci Symp Conf Rec; 2000: IEEE.
- Meier D, Czermak A, Jalocha P, Sowicki B, Kowal M, Dulinski W et al., editors. Silicon detector for a Compton camera in nuclear medical imaging. Nucl Sci Symp Conf Rec; 2000: IEEE.
- Ding Y, Lee J, Ma J, Sung K, Yokota T, Singh N et al. Light-sheet fluorescence imaging to localize cardiac lineage and protein distribution. Sci Rep. 2017;7:42209.
- Ding Y, Xie H, Peng T, Lu Y, Jin D, Teng J et al. Laser oblique scanning optical microscopy (LOSOM) for phase relief imaging. Opt Express. 2012;20(13):14100-8.
- Ding Y, Zhang Y, Peng T, Lu Y, Jin D, Ren Q et al. Observation of mesenteric microcirculatory disturbance in rat by laser oblique scanning optical microscopy. Sci Rep. 2013;3:1762.
- Fei P, Lee J, Packard RRS, Sereti K-I, Xu H, Ma J et al. Cardiac light-sheet fluorescent microscopy for multi-scale and rapid imaging of architecture and function. Sci Rep. 2016;6:22489.
- Liu Y, Ding Y, Alonas E, Zhao W, Santangelo PJ, Jin D et al. Achieving λ/10 resolution CW STED nanoscopy with a Ti: sapphire oscillator. PloS One. 2012;7(6):e40003.
- Peng T, Xie H, Ding Y, Wang W, Li Z, Jin D et al. CRAFT: Multimodality confocal skin imaging for early cancer diagnosis. J Biophoton. 2012;5(5‐6):469-76.
- Xi P, Xie H, Liu Y, Ding Y. Optical nanoscopy with stimulated emission depletion. Opt Nanoscopy Novel Microscopy Tech. 2014:1-22.
- Xie H, Jin D, Yu J, Peng T, Ding Y, Zhou C et al. Schlieren confocal microscopy for phase-relief imaging. Opt Lett. 2014;39(5):1238-41.
- Ding Y, Xi P, Ren Q. Hacking the optical diffraction limit: Review on recent developments of fluorescence nanoscopy. Chin Sci Bull. 2011;56(18):1857-76.
- Vuletic I, Liu J, Wu H, Ding Y, Lei Y, Li C et al. Establishment of an mKate2-expressing cell line for non-invasive real-time breast cancer in vivo imaging. Mol Imaging Biol. 2015;17(6):811.
- Vuletic I, Zhou K, Li H, Bai H, Meng X, Zhu S et al. Validation of Bevacizumab Therapy Effect on Colon Cancer Subtypes by Using Whole Body Imaging in Mice. Mol Imaging Biol. 2017.
- Wang G, Zhang B, Ding Y, He Y, Chen J, Lu Y et al. A modularly designed fluorescence molecular tomography system for multi-modality imaging. J X-ray Sci Technol. 2015;23(2):147-56.
- Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin αvβ3 in brain tumor xenografts. Canc Res. 2004;64(21):8009-14.
- Chudakov DM, Verkhusha VV, Staroverov DB, Souslova EA, Lukyanov S, Lukyanov KA. Photoswitchable cyan fluorescent protein for protein tracking. Nat Biotechnol. 2004;22(11):1435-9.
- Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23(3):313-20.
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22(12):1567-72.
- Zhang S, Ma C, Chalfie M. Combinatorial marking of cells and organelles with reconstituted fluorescent proteins. Cell. 2004;119(1):137-44.
- Hoffman RM. Visualization of GFP-expressing tumors and metastasis in vivo. Biotechniques. 2001;30(5):1016-27.
- Ntziachristos V, Tung C-H, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med. 2002;8(7):757-61.
- Liu JT, Mandella MJ, Ra H, Wong LK, Solgaard O, Kino GS et al. Miniature near-infrared dual-axes confocal microscope utilizing a two-dimensional microelectromechanical systems scanner. Opt Lett. 2007;32(3):256-8.
- Ge J, Erickson SJ, Godavarty A. Fluorescence tomographic imaging using a handheld-probe-based optical imager: extensive phantom studies. Appl Opt. 2009;48(33):6408-16.
- Heaviside O. XXXIX. On the electromagnetic effects due to the motion of electrification through a dielectric. The London, Edinburgh, and Dublin Phil Mag J Sci. 1889;27(167):324-39.
- Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol. 2009;54(16):N355.
- Liu H, Ren G, Miao Z, Zhang X, Tang X, Han P et al. Molecular optical imaging with radioactive probes. PloS One. 2010;5(3):e9470.
- Deshpande N, Needles A, Willmann JK. Molecular ultrasound imaging: current status and future directions. Clin Radiol. 2010;65(7):567-81.
- Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to α v-integrins. Circulation. 2003;107(3):455-60.
- Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107-12.
- Klibanov AL, Rasche PT, Hughes MS, Wojdyla JK, Galen KP, Wible Jr JH et al. Detection of individual microbubbles of ultrasound contrast agents: imaging of free-floating and targeted bubbles. Invest Radiol. 2004;39(3):187-95.
- Behm CZ, Lindner JR. Cellular and molecular imaging with targeted contrast ultrasound. Ultrasound. 2006;22(1):67-72.
- Willmann JK, Lutz AM, Paulmurugan R, Patel MR, Chu P, Rosenberg J et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248(3):936-44.
- Ding Y, Ren Q, Li C. Advanced photoacoustic microscopy. Opt Nanoscopy Novel Microscopy Tech. 2014:215.
- Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335(6075):1458-62.
- Zhang HF, Maslov K, Stoica G, Wang LHV. Imaging acute thermal burns by photoacoustic microscopy. J Biomed Opt. 2006;11(5):054033.
- Hu S, Wang LV. Optical-Resolution Photoacoustic Microscopy: Auscultation of Biological Systems at the Cellular Level. Biophys J. 2013;105(4):841-7.
- de la Zerda A, Paulus YM, Teed R, Bodapati S, Dollberg Y, Khuri-Yakub BT et al. Photoacoustic ocular imaging. Opt Lett. 2010;35(3):270-2.
- Jiao S, Jiang M, Hu J, Fawzi A, Zhou Q, Shung KK et al. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt Express. 2010;18(4):3967-72.
- Jiao S, Xie Z, Zhang HF, Puliafito CA. Simultaneous multimodal imaging with integrated photoacoustic microscopy and optical coherence tomography. Opt Lett. 2009;34(19):2961-3.
- Razansky D, Baeten J, Ntziachristos V. Sensitivity of molecular target detection by multispectral optoacoustic tomography (MSOT). Med Phys. 2009;36(3):939-45.
- Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nat Photon. 2009;3(9):503-9.
- Kim J-W, Galanzha EI, Shashkov EV, Moon H-M, Zharov VP. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat Nanotechnol. 2009;4(10):688-94.
- Pramanik M, Song KH, Swierczewska M, Green D, Sitharaman B, Wang LV. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Phys Med Biol. 2009;54(11):3291.
- Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano. 2010;4(8):4559-64.
- Nahrendorf M, Keliher E, Marinelli B, Waterman P, Feruglio PF, Fexon L et al. Hybrid PET-optical imaging using targeted probes. Proc Natl Acad Sci. 2010;107(17):7910-5.
- Beyer T, Townsend DW, Brun T, Kinahan PE. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41(8):1369.
- Mawlawi O, Townsend DW. Multimodality imaging: an update on PET/CT technology. Eur J Nucl Med Mol Imaging. 2009;36:15-29.
- Pichler BJ, Wehrl HF, Judenhofer MS. Latest advances in molecular imaging instrumentation. J Nucl Med. 2008;49(Suppl 2):5S-23S.
- Li S, Meng X, Zhou K, Xie Z, Ding Y, Yang K et al. Overview of Advances in Molecular Imaging (Part1). China Med Dev. 2015;30(2):1-6.
- Lu Y, Yang K, Zhou K, Pang B, Wang G, Ding Y et al. An integrated quad-modality molecular imaging system for small animals. J Nucl Med. 2014;55(8):1375-9.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/