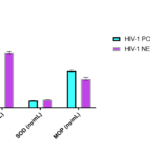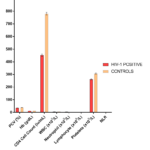J Med Discov (2017); 2(2):jmd17025; DOI:10.24262/jmd.2.2.17025; Received June 11th, 2017, Revised July 4th, 2017, Accepted July 5th,2017, Published July 20th, 2017.
Association of Neutrophil-to-lymphocyte ratio with Respiratory burst enzymes in Human Immunodeficiency virus type 1 infected Africans
Mathias Abiodun Emokpae1,*, Austin Iroghama Aruomaren1,Beatrice Aghogho Mrakpor1
1Department of Medical Laboratory Science School of Basic Medical Sciences College of Medical Sciences University of Benin Benin City Nigeria.
*Correspondence:Dr. M.A Emokpae, Department of Medical Laboratory Science, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, Benin City. Email: mathias.emokpae@uniben.edu.
Abstract
The hallmark of human immunodeficiency virus type 1 (HIV-1) infection is the progressive depletion of cluster of differentiation (CD4+) T cell populations, aberrant activation of immune system and inflammatory response. This study looked at the association between neutrophil-to- lymphocyte ratio (NLR) and respiratory burst enzymes in HIV-1infected Africans. The study participants comprised of 150 (50 males and 100 females) HIV infected subjects and 50 (25 males and 25 females) HIV-1 negative volunteers as controls. Complete blood count was analyzed using automated ERMA auto-analyzer PCE-210N, (Diamond Diagnostics, Holliston, USA). NLR was calculated from the results of absolute values of neutrophil and lymphocyte. Catalase, superoxide dismutase and myeloperoxidase activities were assayed using kits supplied by Wkea Medical supplies Corp., Changchun, China. The measured parameters in HIV-1 positive subjects were compared with controls using unpaired t-test while Pearson correlation coefficient was used for correlation. Superoxide dismutase activity correlated inversely with NLR (r=-.186; p<0.01) and positively CD4 count (Pearson r=0.439, p<0.001). Serum catalase (p<0.05) and myeloperoxidase (p<0.01) activities were significantly higher while superoxide dismutase activity was significantly lower (p<0.01) in HIV infected subjects than controls. A significantly higher (p<0.01) level of NLR was observed in HIV infected subjects than controls. In conclusion, NLR was higher while superoxide dismutase activity was lower in HIV-1 positive subjects than controls which also correlated with CD4+cells. Therefore, NLR may be used routinely to monitor inflammatory status and superoxide dismutase activity as an indicator of oxidative stress in HIV infected subjects.
Keywords: Human Immunodeficiency virus type 1, catalase, myeloperoxidase, superoxide dismutase
Introduction
During the course of Human Immunodeficiency virus (HIV) infection, polymorphonuclear leukocytes (PMNs) and interleukins are usually the first line of defense of innate immune in response to the disease [1]. Polymorphonuclear leukocytes exhibit a variety of immunologic and functional defects that may contribute to increased susceptibility of host to pathogens [2]. Patients with HIV infection display a variety of immunologic defects that almost invariably lead to the development of a diverse array of opportunistic infections and during the course of the disease. Studies have shown dysfunctional activity of PMNs in patients with HIV/AIDS in their chemotactic, phagocytic and oxidative metabolism processes [3,4]. Particularly, decreased chemotaxis and killing activity, as well as altered respiratory burst response and accelerated apoptosis have been reported [5-7].
Respiratory burst, is characterized by the conversion of oxygen to several toxic products, including superoxide anion and hydrogen peroxide, which are important inflammatory activities in PMNs [8]. Chemoattractant-receptor coupling triggers several biologic responses in phagocytic cells including activation of the respiratory burst. Prior evidence in intact cells implied that stimulation of the respiratory burst by chemoattractants was by a mechanism different from other soluble agents suggesting the possibility that different oxidative enzymes were responsible [9]. Interleukin (IL)-15 has been reported to be a chemoattractant for T lymphocytes, natural killer
(NK) cells and to induce lymphokine-activated killer (LAK) activity in NK cells, as well as be able to promote the generation of cytolytic effector cells [10]. Interleukin-15 is an important cytokine in the activation of the functional properties of HIV positive PMNs, by delaying apoptosis and enhancing chemotaxis activity [1].The activated neutrophils release myeloperoxidase (MPO), a specific enzyme with strong oxidative activity and the plasma MPO activity may be a marker of the neutrophil proliferation and severity of inflammation [9].
Cluster of differentiation (CD4+) T cells play a key role in controlling HIV-1 replication and progression to AIDS [11]. However, HIV-1 infection is associated with a progressive loss of T cell functional capacity including decreased responsiveness to antigenic stimuli, lowered capacity to produce cytokines, and reduced proliferative and cytotoxic activity [12,13]. Previous studies have associated neutrophil dysfunction and decreased superoxide radicals with severity of HIV-1 infected subjects [14,15],oxidative burst enzymes activities [16] and the non-existence of sex differences in the activities of respiratory burst enzymes in HIV-1 infection [17]. Evidence of the relationship between NLR and respiratory burst enzyme in HIV-1 infected Africans is scare in literature. Therefore, this present study aims at evaluating the association between NLR, respiratory burst enzymes and CD4+ count in HIV-1 infected Africans.
Materials and Methods
Selection of Participants
The study participants were consecutively recruited and comprised of 200 subjects that consisted of 150 confirmed HIV-1 positive individuals receiving highly active antiretroviral therapy (HAART) (50 males with mean age of 35.4± 0.5 years and 100 females with mean age of 32.5± 0.4 years) and 50 HIV-1 negative (apparently healthy) individuals recruited from among staff and students of our institution (controls, 25 males with mean age of 34.6± 0.2 years and 25 females with mean age of 32.4 ±0.6 years).
Inclusion and Exclusion Criteria
All the confirmed HIV-1 subjects attending the antiretroviral therapy (ART) clinics at the Central Hospital, Benin City that gave consent were included in the study.
All HIV-1 seronegative subjects who had an illness or infection such as chest infections, bacterial endocarditis or smoke cigarettes that may affect respiratory burst enzymes and those who did not give consent were excluded from the study.
Ethical Consideration
The study protocol was reviewed and approved by the Ethics Committee of the Edo State Ministry of Health (ethical code HM.1208/112, dated 12th May,2016), before the commencement of the study. Informed consent was sought and obtained from all cases and volunteers and utmost confidentiality of information were maintained.
Analytical methods
Six milliliters of blood sample were collected by venous puncture; 3 mL were dispensed into a plain tube, while the remaining 3 mL were emptied into bottles containing ethylene diamine tetra-acetic acid (EDTA). The sample in the plain container was allowed to clot at room temperature. The clotted sample was centrifuged at 3000 rpm for 10 min. The serum was separated into plain containers and stored at -20oC before it was analysed. The serum MPO, SOD and catalase (CAT) were assayed by Enzyme Linked Immunosorbent Assay (ELISA) technique using reagent kits supplied by Wkea Medical supplies Corp.,Changchun, China. The experiments were done according to the manufacturer’s protocol.The CD4+ count was estimated using Fluorescence Activated Cell Sorter (Facs Flow Cytometer) count system, Lincolnshire, IL, USA.
Complete blood count analysis
The full blood count was analyzed within four hours of collection of samples using the automated ERMA Haematology auto-analyzer PCE-210N (Diamond Diagnostics, Holliston, USA). Neutrophil-to-lymphocyte ratio was calculated by dividing the value of absolute Neutrophil count by absolute lymphocyte count.
Data Analysis
All data were presented as mean ± S.E.M. Unpaired t-test (two groups) and correlation analysis were performed by statistical software (SPSS version 21, IBM, IL, USA). Value was considered to be statistically significant at p < 0.05.
Results
In the comparison of measured respiratory burst enzymes between HIV-1 positive subjects and controls, serum catalase (p<0.05) and MPO(p<0.001) activities were significantly higher in HIV-1 positive subjects, while SOD activity was significantly lower (p<0.001) in HIV-1 positive subjects than control (Tab. 1 & Fig. 1).
| Measured Parameters | HIV-1 Positive Subjects | HIV-1 Negative Subjects | p-value |
| Number of Subjects | 150 | 50 | |
| Catalase (iu/L) | 16.0 ± 0.82 | 11.8 ± 0.49 | < 0.05 |
| SOD (ng/mL) | 1.61 ± 0.02 | 1.75 ± 0.03 | < 0.001 |
| MPO (ng/mL) | 7.90 ± 0.48 | 6.20 ± 0.55 | < 0.001 |
Table 1. Comparison of Respiratory burst enzymes in HIV-1 infected subjects Note: SOD=superoxide dismutase; MPO= myeloperoxidase
Figure 1. Comparison of Respiratory Burst enzymes in HIV-1 infected subjects.
The mean haematocrit (PCV), haemoglobin (Hb), total white blood cell count, platelet count, absolute neutrophil and lymphocyte as well as CD4+ cell count were significantly lower (p<0.001) in HIV-1 positive compared with controls. Neutrophil-to-lymphocyte ratio (NLR) was higher (p<0.001) in HIV-1 infected subjects when compared to controls (Tab. 2 & Fig. 2).
| Parameters | HIV-1 (n=150) | Controls (n = 50) | p value |
| Packed Cell Volume (%) | 35.5 ± 0.32 | 38.8 ± 0.50 | < 0.001 |
| Haemoglobin (g/dL) | 11.8 ± 0.11 | 13.7 ± 0.09 | < 0.001 |
| CD4+ Count (iu/mL) | 452.5 ± 15.8 | 780.9 ± 19.6 | < 0.001 |
| WBC (X109/L) | 4.77 ± 0.11 | 5.95 ± 0.15 | < 0.001 |
| Neutrophil (X109/L) | 3.75 ± 0.09 | 2.51 ± 0.08 | < 0.001 |
| Lymphocyte (X109/L) | 1.93 ± 0.05 | 2.14 ± 0.07 | <0.001 |
| Platelet Count (X109/L) | 262.04 ± 7.4 | 307.8 ± 8.57 | < 0.001 |
| NLR | 1.93 ± 0.15 | 1.16 ± 0.14 | < 0.001 |
Table 2. Comparison of Haematological indices in HIV-1 infected subjects with controls
Figure 2. Comparison of some Haematological indices in HIV-1 infected subjects with controls.
Table 3 shows a correlation between respiratory burst enzymes, NLR and CD4 cell count in HIV-1 positive subjects and controls. The data showed a negative but significant correlation between superoxide dismutase and CD4+count in HIV-1 positive subjects (p<0.001). Catalase and myeloperoxidase activities had a no-significant (p>0.05) negative correlation with CD4 cell count in HIV-1 positive subjects.
The correlation between NLR and respiratory burst enzymes in HIV-1 positive subjects and controls showed a significant correlation between SOD and NLR (r-0.189;p<0.01) in HIV-1 positive subjects while SOD correlated positively with CD4+ count (r0.439;p<0.001) (Tab. 3 & Tab. 4).
| Correlation | R-Value | p value |
| HIV Infected Subjects | ||
| CAT vs CD4 | -0.16 | 0.267 |
| SOD vs CD4 | 0.439 | <0.001 |
| MPO vs CD4 | -0.188 | 0.19 |
| HIV Negative Subjects | ||
| CAT vs CD4+ | -0.206 | 0.20 |
| SOD vs CD4+ | 0.188 | 0.20 |
| MPO vs CD4+ | 0.099 | 0.50 |
Table 3. Correlation of Respiratory Burst enzyme with CD4+ Count in HIV-1 infected subjects and controls
| Correlation | R-Value | p value |
| HIV Infected Subjects | ||
| CAT vs NLR | -0.078 | 0.262 |
| SOD vs NLR | -0.186 | <0.01 |
| MPO vs NLR | 0.065 | 0.346 |
| HIV Negative Subjects | ||
| CAT vs NLR | 0.058 | 0.70 |
| SOD vs NLR | 0.086 | 0.55 |
| MPO vs NLR | 0.065 | 0.60 |
Table 4. Correlation of Respiratory Burst enzyme with Neutrophil Lymphocyte ratio in HIV-1 infected subjects and controls
Discussion
The value of NLR increased in HIV-1 positive subjects and correlated negatively with superoxide dismutase activity. The result of this study is consistent with previous study[18]. The authors evaluated the role of NLR and PLR as simple diagnostic inflammatory markers in the diagnosis and severity of HIV infection. They concluded that increase in NLR, which was common in most HIV subjects were associated with increasing risk of death in most of the subjects. Postorino et al.[19] also reported a similar finding in an Italian Master cohort study. NLR is a readily measurable laboratory marker used to evaluate systemic inflammation. Numerous studies have demonstrated aberrant inflammatory response and immune action in HIV infection and these inflammatory responses have been shown to play critical role in the pathogenicity of the disease [20-22]. Deeks et al.[23] reported that HIV replication contributes directly to T cell activation, breakdown of gut mucosa and chronic exposure of gut microbial products are key factors driving inflammation.
Neutrophils are major producers of ROS and cytotoxic activity of neutrophils depends on very intricate mechanisms including the release of proteolytic enzymes and the rapid production of ROS, which act as defense against foreign pathogens [24]. ROS could affect DNA, proteins or lipid structures thereby altering their intrinsic abilities resulting in apoptotic cell death [25]. The observation of significantly higher catalase and myeloperoxidase enzymatic activities suggests that HIV patients are predisposed to increased oxidative stress. This is also in agreement with previous report [16,26,27]. Myeloperoxidase is considered virucidal in HIV-1 infected subjects[28]. Superoxide dismutase was significantly lower in HIV-1 positive subjects than HIV seronegative subjects. Our finding is in agreement with previous studies [29,30]. The authors reported a decrease in superoxide dismutase activity in HIV seropositive subjects when compared with HIV negative subjects in Southeast Nigeria. The higher catalase activity we observed in HIV-1 positive subjects was however not consistent with other studies [29,30]. We previously reported a no-sex difference in the activities of respiratory burst enzymes in HIV-1 infected subjects, but the measured enzyme activities were significantly higher in females than males in HIV-1 negative individuals [17].
Our study observed a negative significant correlation between SOD, NLR and CD4. Similarly, Ibeh et al.[30] and Gwarzo et al.[31] reported a negative significant correlation between SOD and CD4. A no significant negative correlation that was observed in this study did not agree with that of El-Bejjani et al. [27], which reported a negative association between MPO activity and CD4 cell count.
Lower CD4+ cell count has been associated with HIV infection. The mechanism of depleted CD4+ cell in HIV infection has been attribute to immune failure and the ability of virus to deplete CD4+ T cell subsets [32], other authors also reported that HIV virus replication site in the gastrointestinal tract, resulting inflammatory response and apoptosis are other possible causes of CD4+ T cell depletion [33]. Anaemia is one of the most common haematological abnormalities in HIV infected subjects. The low haematocrit values reported in this study, is in accordance with other studies [34,35]. The result also showed a significant increase in neutrophil in HIV-1 subjects. However, Santis et al. [34] reported neutropenia in HIV infected subjects. Neutrophil usually has a life-span of 24hours after been released from the bone marrow [36]. This neutrophil apoptosis can affect the number of circulating neutrophil and the subsequent survival of these cells. Apoptosis had been linked with inhibition of reactive oxygen species (ROS) and the activities of caspase 3 [37]. However, neutropenia was reported in chronic phase of the disease and related to disease progression to AIDS. None of the subjects in this study had AIDS.
Conclusion
This study reported higher level of NLR which correlated inversely with SOD and CD4+ in HIV-1 infected subjects which may be due to increase inflammatory activity and oxidative stress in infected subjects. Calculation of NLR as biomarker of inflammation may be routinely done in the management of HIV-1 infected subjects.
Acknowledgments
We appreciate the contributions of all staff of the Central Hospital, Benin City, Edo State, Nigeria. We are grateful to the Medical Laboratory Scientists for their technical support.
Author Contributions
Mathias Abiodun Emokpae conceived, designed the experiments, performed the analysis and wrote the manuscript; Beatrice Aghogho Mrakpor performed the data gathering and analysis Austin Iroghama Aruomaren assisted with statistical analysis and writing of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
1. Mastroianni, C M, d´Ettorre, G., Forcina G, Lichtner M, Mengoni F et al. Interleukin-15 enhance neutrophil functional activity in patients with HIV infection. Blood. 2000; 96: 1979–1884.
2. Shyur SD and Hill HR. Polymorphonuclear leucocyte function in HIV. In Gupta S. ed. Immunology of HIV infection. New York, Plenum Press.1996 p. 377–386.
3. Elbim C, Prevot MH, Bouscarat F et al. Polymorphonuclear neutrophil from HIV infected patients with endonuclease activation diminished FMLP-induced L-selectin shedding an impaired oxidative burst after cytokine priming. Blood. 1994;84(8): 2759–2766.
4. Michailidis C, Giannopolos G, Vigklis V, Armenis K, Tsakris A, Gargalianos A. Imparedphargocytosis among patients infected by the HIV: Implication for the role of HAART. Clin Experiment Immunol 2012;167(3): 499–504.
5. Elbim C and Lizard G. Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situation. Cytometry A. 2009;75(6): 475–481.
6. Elbim C, Monceaux V, Francois S, Hurtrel B, Gougerot-Pocidalo MA, Estaquier J. Increased neutrophil apoptosis in chronically SIV-infected macaques. Retrovirology 2009; 24: 6-29.
7. Lichtner M, Mengoni F, Mastroianni CM,Sauzullo I, Rossi R, DeNicola M et al. HIV protease inhibitor therapy reverses neutrophil apoptosis in AIDS patients by direct calpain inhibition. Apoptosis 2006; 11(5): 781–787.
8. McPhail L and Sryderman R. Activation of the respiratory burst enzyme in human polymorphonuclear leukocyte by chemoattractants and other stimuli: Evidence that the same oxidase is activated by different transduction mechanism.J Clin Invest. 1983;72(1):192–200.
9. Kothari N, Keshari RS, Bogra J,Kohli M, Abbas H, Malik A et al. Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J Crit Care. 2011; 26(4): 435e1 – 435e7.
10. Forman HJ and Torres M. Signaling by the Respiratory Burst in Macrophages. IUBMB Life 2001; 51: 365–371.
11. Bower NL, Helton ES, Huijbregts RPH, Goepfert PA, Heath S, Hel Z. Immune suppression by neutrophils in HIV-1 infection: Role of PDL1/PD-1 pathway. PLosPathog.2014; 10(3): e1003993.
12. Hel Z, McGhee JR, Mestecky J. HIV infection: First battle decides the war. Trends Immunol. 2006; 27: 274 – 281.
13. Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE et al. Expression of CD57 defines replicative senescene and antigen – induced apoptotic dealth death of CD8 T cells. Blood. 2003;101: 2711 – 2720.
14. Munoz JF, Salmen S, Berrueta LR. Effect of HIV-1 on intracellular activation and superoxides production by neutrophils. J. Infect. Dis. 1999;180: 206 – 210.
15. Gabrilovich DI, Vassilev V, Nosikov VV, Serebrovskaya LV, Ivanova LA, Pokrovsky VV. Clinical significant of HIV DNA in polymorphonuclear neutrophil frpom patients with HIV infection. J. Acquir. Immune. Defic. Syndr. 1993;6: 587 – 591.
16. Emokpae MA, Mrakpor BA. Serum activities of oxidative stress burst enzymes in Human Immunodeficiency Virus infected subjects. HIV AIDS Rev 2017;16(2):1-5.
17. Emokpae MA, Mrakpor BA. Do Sex Differences in Respiratory Burst Enzyme Activities Exist in Human Immunodeficiency Virus-1 Infection? Med Scis 2016;4(19):1-8.
18. Raffetti E, Donato F, Casari S,Castelnuovo F, Sighinolfi L, Banedera A et al. Systemic inflammation based scores mortality for all causes in HIV infected patients: a Master Cohort study. BMC Infect Dis 2017 doi: 1186/s12879-017-2280-5.
19. Postorino MC, Prosperi M, Foca E,Quiros-Roldan E, Maggiolo F, Borghetti A et al. Role of systemic inflammation scores for prediction of clinical outcomes in patients treated with atazanovir not boost by ritonavir in the Italian Master Cohort. BMC Infect Dis 2017;17(1): 212 –215.
20. Erlandson KM and Campbell TB. Inflammation in chronic HIV infection: what can we do. J Infect Dis 2015; 212(3): 339 – 342.
21. Ipp H, Zemlin AE, Erasmus RT, Glashoff RH. Role of inflammation in HIV-1 disease progression and prognosis. Crit. Rev. Clin. Lab. Sci. 2014; 51(2): 98 – 111.
22. Kuller LH, Tracy R, Belloso W, DeWit S, Drummond F, Lane HC. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLos Med. 2008;5(10): e203.
23. Deck SG, Tracy R, Douek DC. Systemic effect of inflammation in health during chronic HIV infection. Immunity.2013; 39(4): 633 – 645.
24. Mantovani A, Cassetalla MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11(9): 519 – 531.
25 Isaguliant M, Smirnova O, Ivanov A,Kilpelainen A, Kuzmenko Y,Petkov S et al. (2013) Oxidative stress induced by HIV-1 reverse transcriptase modulates the enzyme performance in gene immunization. Hum vaccines Immunotherapeut. 2013;9(10): 2111 – 2119.
26. Allard JP, Aghadassi E, Chau J, Salit I, Walmsley S. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. Am J Clin Nutr 1998; 67(1): 143 – 147.
27. El-Benjjani D, Hazen SL, Mackay W, Glass NE, Hulgan T, Tungsiripat M, McComsey GA. Higher plasma myeloperoxidase levels are not associated with an increased risk for cardiovascular events in HIV-infected adults. HIV Clin. Trials. 2008; 9(3): 207 – 211.
28. Chochola J, Yamaguichi Y, Moguilevsky N, Bollen A, Strosberg AD, Stanislawski M. Virucidal effect of myeloperoxidase on human immunodeficiency virus type 1 –infected cells. Antimicrob. Agents. Chemotherapy. 1994;38(5): 969 – 972.
29. Aniagolu MO, Ezeanyika S, Parker J, Shu N, Ngwu AM, Ikegwuonu Changes in some antioxidants in HIV positive patients on highly active antiretroviral therapy (Case study of Nsukka, South East Nigeria). Asia J SciTechnol 2015; 6(4): 1289 – 1292.
30. Ibeh BO, Habu JB, Eze SC. Discordant levels of superoxide dismutase and catalase observed in ART naïve and experienced HIV patients in South Eastern Nigeria. J Infect Dis. 2013;1: 8 – 16.
31. Gwarzo MY and Muhammad SA. Extracellular superoxide dismutase activity and plasma malondialdehyde in HIV subjects of Kano State as surrogate markers of CD4 status. Int. J. Biomed. Sci. 2010;6(4): 294 – 300.
32. Okoye AA and Picker LJ. CD4+ T cell depletion in HIV infection: Mechanism of immunological failure. Immunol. Rev. 2013; 254(1): 54 – 64.
33. Felrier M, Dorghan K,Rebollo A. CD4+ T cell depletion in HIV infection: role of apoptosis. Virus. 2011; 3(5): 586 – 612.
34. Santis GC, Brunetta DM, Vilar FC,Brandao RA, Muniz RZ, de Lima MN, Amorelli-Chacei ME et al. Haematological abnormalities in HIV-infected patients. Int. J.Infect. Dis. 2011; 15: 808 – 811.
35. Abram DI, Steinhart C, Francino R. Epoetinalfa therapy for anaemia in HIV-infected subjects: Impact on the quality of life. Int. J. STD AIDS. 2000;11: 659 – 665.
36. Pitrak DL. Apoptosis and role in neutrophil dysfunction in AIDS. The Oncologist. 1997;2(2): 121 – 124.
37. Salmen S, Montes H, Sayono A, Hernandez D, Berrueta L. Mechanism of neutrophil death in HIV – infected patients: Role of oxygen species caspase and MAP kinase pathway. Clin Experiment Immunol 2007;150: 539 – 545.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/