J Med Discov (2017); 2(2):jmd17015; DOI:10.24262/jmd.2.2.17015; Received April 19th, Revised April 30th, Accepted May 1st, Published May 21st.
Development of NDDS of Carbamazepine in Epilepsy for Medical Discovery of Formulation Significance
Gannu Praveen Kumar1,*, D.Rambhau2, S.S.Apte3
1.2.3University College of Pharmaceutical Sciences, Kakatiya University, Warangal – 506009, Telangana, India
* Correspondence: Gannu Praveen Kumar, Sahasra Institute of Pharmaceutical Sciences, Warangal – 506007, Telangana, India. Email: ghalo2010@gmail.com.
Abstract
Novel Drug Delivery Systems (NDDS) such as oral cationic, anionic, nonionic self nanoemulsifying drug delivery systems (SNEDDS) and self macroemulsifying drug delivery systems (SMEDDS) incorporating carbamazepine were developed by the optimization of various USFDA approved excipients like oils, surfactants, cosolvents, and lipids. Oleic acid was included as the oil, Labrasol was the primary surfactant and lecithin as a secondary surfactant. Cetyl pyridinium chloride and 1,2-dioleoyl-3-tri-methyl ammonium propane were used to impart positive charge and Potassium docusate to incorporate negative charge. The SNEDDS and SMEDDS dispersions were stable in all the biorelevant release media (0.1N HCl, phosphate buffer, simulated gastric fluid (SGF), fasting simulated gastric fluid (FASGF), simulated intestinal fluid (SIF), fasting simulated intestinal fluid (FASIF) and fed simulated intestinal fluid (FESIF) for a period of 24hrs and 12hrs which is sufficient enough for complete absorption of the drug. The release of carbamazepine from these SNEDDS was completed (75-94%) within 30mins. Their size ranged from 108-180nm with an entrapment of 76-87% respectively. The SNEDDS were infinitely dilutable without any phase separation. The droplet size obtained with cationic SNEDDS was significantly lower compared to anionic and non-ionic SNEDDS (p<0.001). The entrapment of carbamazepine was highest in cationic SNEDDS (p<0.001).The drug release from all the SNEDDS was rapid and complete within 30mins when compared to SMEDDS and suspension. However, the SMEDDS (whose size ranged from 230-630nm) and suspension could not release not more than 69% and 50% at 30mins. The cationic, anionic and nonionic SNEDDS released about 90%, 81% and 50% of the drug in the initial 15mins, followed by a gradual release of the remaining drug, over the next 30mins. It was also observed that almost the entire drug was released in FASGF, FASIF and FESIF. In cationic SNEDDS the release was probably enhanced due to its size and the effect of charged and physiological surfactants.
Keywords: SEDDS, SNEDDS, SMEDDS, Biorelevant, Carbamazepine, Cationic, Anionic, Non-ionic
Introduction
The preparation of Novel Drug Delivery Systems (NDDS) like nanoemulsions by spontaneous emulsification has emerged as a promising alternative to conventional high energy emulsification [1-3]. Stable nanoemulsion by low energy emulsification suitable for enhancing oral absorption is a boon for pharmaceutical applications. Therefore, self emulsifying nanoemulsions offer an appealing alternative for the administration of poorly water soluble drugs like carbamazepine to their effectiveness for drug solubilisation, potential for improved efficacy, anticipated patient acceptance and compliance due to reduced side effects [4].
Apart from the poor water solubility, extensive first-pass metabolism by Cytochrome P450-3A4 (CYP3A4) isoenzymes and P-glycoprotein (P-gp) mediated efflux are also responsible for it’s low oral bioavailability [5]. However, the clinical efficacy is strongly limited by its poor water solubility and low oral bioavailability [6].
Self Nanoemulsifying Drug Delivery System (SNEDDS) is a novel drug delivery system consisting of active substance/s, oils, co-solvents and surfactants. The gentle mixing of SNEDDS preparation in aqueous media can generate nanoemulsion droplets (with a mean droplet size <200nm) of solubilized drugs [7]. Though the concept of self nanoemulsifying system is fairly old, it is gaining prominence in recent years, particularly to improve the solubility of highly lipophilic bioactives in aqueous media. It is considered that such self nanoemulsifying systems may improve the absorption of drugs by rapid self emulsification in the gastrointestinal tract, with the nanoemulsion droplets subsequently dispersing in the gastrointestinal tract, thereby allowing them to be readily absorbed. They are versatile and offer numerous possibilities to the formulation designers for developing suitable dosage forms to meet the requirements of specific molecules. They are likely to have an increasing application in the design of innovative delivery systems for lipophilic drugs.
Uncontrolled epilepsy is a considerable burden for the patient and may be associated with severe consequences, including shortened lifespan, excessive bodily injury, neuropsychological & psychiatric impairment and social disability [8]. Thus, it is important to determine the reasons for antiepileptic drug failures and to develop novel formulation strategies to overcome obstacles to seizure control.
Carbamazepine is a widely used nonsedative antiepileptic agent which has been effective in the therapy of psychomotor seizures and trigeminal neuralgia for 40 years [9-11]. Due to its poor aqueous solubility (<200mcg/mL), the drug exhibits unpredictable and erratic absorption profile [12-14]. Currently, carbamazepine is available in tablets (immediate release, controlled release and chewable), capsule and oral suspension formation. None of the formulations are reported to control the erratic absorption profile and enhance the solubility in the gastrointestinal fluids. Thus, it is an emergency to develop novel strategies to design new delivery systems. Among them, Self Emulsifying Drug Delivery Systems (SEDDS) [15, 16] which produces nanoemulsions on mild agitation is one of the novel drug delivery system to improve solubility and is of current interest. The objective was to reduce the dose of carbamazepine and enhance the solubility thereby to achieve similar plasma levels.
Materials and Methods
Reagents
Oleic acid, Ethyl oleate, Ethyl Linoleate, Medium chain triglycerides, Soya bean oil, Triolein, Isopropylmyristate, Cetyl pyridinium chloride, Potassium docusate and 1,2-dioleoyl-3-tri-methyl ammonium-propane, sodium taurocholate (99% pure), sodium lauryl sulfate, calcium chloride dihydrate, Pepsin, Pancreatin, sodium chloride, sodium hydroxide were purchased from Sigma Aldrich Co. (St. Louis, MO, USA)., Tocopheryl polyethylene glycol succinate 1000 was a gift sample from Eastman chemicals UK; carbamazepine was gifted by Sun Pharmaceuticals, Span80, Polyethylene glycol-200, Hydrochloric acid and potassium dihydrogen phosphate were purchased from Merck Ltd; Labrasol (C8/C10 polyglycolyzed glycerides from coconut oil) was a gift sample from Colorcon laboratories Goa, and lecithin (Lipoid S PC, 98% phosphatidylcholine) was a generously gifted by Lipoid GmbH, Ludwigshafen, Germany and water was obtained from NanoPure purification system. All other chemicals were of analytical grade and were used as supplied without further modification.
Solubility study
To develop an optimized SNEDDS of CBZ, various clinically useful oils were screened to determine the solubility. Some of the oils selected for determining the solubility are medium chain triglycerides (MCT), soya bean oil (SBO), oleic acid (OA), triolein (TRIO), ethyl lionoleate (EL), ethyl oleate (EO) and isopropyl myristate (IPM). To a 15ml culture tube containing 5g of oil or oil mixture, excess quantity of carbamazepine (CBZ) was added and the contents were dissolved in methanol/chloroform (1:1) mixture. The solvent mixture was vortexed for 1-3 minutes. The homogeneous solvent mixture was transferred to a 100ml evaporating flask and fixed to rotary evaporator (Buchi, switzerland). The solvent was vaccum evaporated at 50°c and 57mbar. The oily solution saturated with CBZ was allowed to stand at room temperature (RT) 32°c for 24hrs and CBZ crystals formed in the oil were separated by ultracentrifugation of oil at 100,000rpm (micro centrifuge sartorious, USA). The oil solubility of CBZ was determined by analysing the CBZ content in supernatant oil phase using UV spectroscopy.
Composition of oral SMEDDS and SNEDDS
SNEDDS (cationic, anionic and nonionic) and SMEDDS for oral delivery containing oleic acid as oil; span 80, lecithin, labrasol as surfactants, PEG 200 as cosolvent, cetyl pyridinium chloride & DOTAP as cationic and potassium docusate as anionic charge inducers and TPGS as nonionic surfactant were developed by optimizing the concentration of components in varied weight ratios. The optimized formulae of SMEDDS, SNEDDS (cationic, anionic and nonionic) is shown in Table 1.
| Ingredients | SMEDDS
(%) |
SNEDDS (%) | ||
| SNEDDS A | SNEDDS B | SNEDDS C | ||
| CBZ | 100mg | 100mg | 100mg | 100mg |
| OA | 11.11 | 5.95 | 5.40 | 5.52 |
| SPAN 80 | 10 | 11.90 | 10.81 | 6.63 |
| LECITHIN | – | 1.19 | 1.08 | 1.66 |
| LABRASOL | 67.78 | 72.62 | 65.95 | 67.40 |
| TPGS | 5.56 | 5.95 | 5.40 | 7.73 |
| PEG 200 | – | 5.95 | 5.40 | 5.52 |
| DOTAP | – | 0.59 | – | – |
| CPC | – | 1.79 | – | – |
| K.DOC | – | – | 0.54 | – |
Table 1. SMEDDS and SNEDDS dispersions
Note: SMEDDS - self macroemulsifying drug delivery system; SNEDDS - self nanoemulsifying drug delivery system; CBZ – carbamazepine; OA – oleic acid; TPGS – tocopheryl polyethylene glycol succinate 1000; DOTAP - 1,2-dioleoyl-3-tri-methyl ammonium-propane; CPC – cetyl pyridinium chloride; K.Doc – potassium docusate
Preparation of carbamazepine SEDDS (SNEDDS & SMEDDS)
In accordance with the general composition in the preparation of SEDDS of CBZ, we have chosen oleic acid in combination with various listed oils to determine the maximum solubility in these oils. Labrasol because of its high HLB value was considered as a prime hydrophilic surfactant for ease of dispersion and to enhance the solubility. Other surfactants such as span 80, lecithin and TPGS were also included in the formulations to improvise the stability of SNEDDS dispersion. PEG 200 was chosen as cosolvent to improve the solubility and charged surfactants such as CPC for imparting positive charge & to augment the positive charge magnitude DOTAP was also included and potassium docusate for imparting negative charge. To improve the ease of dispersion, solvents like ethanol and benzyl alcohol were also included. The optimization of the composition for oral SNEDDS of CBZ was carried out by varying the weight ratios between the oils and surfactants and an optimal oral SNEDDS (cationic, anionic and non-ionic) of CBZ were developed. While varying such ratios between various components, the rest of the composition was kept constant althroughout. All the resultant formulations were evaluated for ease of dispersion, size, zeta potential, appearance, stability and turbidity. Nanosystems containing diclofenac sodium were prepared by flash evaporation method. Oils, lipophilic surfactants and the drug were dissolved in 1.5ml of 1:1 mixture of methanol and chloroform in clean and previously dried 15ml culture tube. The remaining ingredients i.e; hydrophilic surfactants and cosolvents were dissolved in another 1.5ml of 1:1 mixture of methanol and chloroform. Both the solutions were transferred to 100ml round bottom flask. Further the culture tubes were rinsed with the remaining 1ml of 1:1 mixture of methanol and chloroform. The oil solution was flash evaporated at 50°c at 110rpm for 20 minutes. The oil solution was finally transferred into a screw capped bottle and was observed for any signs of crystallization.
Size and Zeta potential
The SEDDS preparations were diluted with 0.1 micron filtered biorelevant release media.. Droplet size was determined by photon correlation spectroscopy using a Zetasizer 3000 HAS / Zetasizer ZS-90 (Malvern Instruments,UK). Light scattering was monitored at 25 °C at a 90° angle. The mean size and standard deviation (± S.D) was directly obtained from the instrument.
Percent encapsulation
The percent encapsulation of carbamazepine was determined by measuring the concentration of free drug in the dispersion medium. Centrifugal ultrafiltration was carried out using Centrisart I (Sartorius AG, Gottingen, Germany) which consists of filter membrane (molecular weight cut-off, 20000 Da) at the base of the sample recovery chamber. The amount of carbamazepine in the aqueous phase was estimated. This was taken as unencapsulated drug in the preparation. Total drug was analyzed after digesting the system with methanol. The percent encapsulation was calculated as follows:
The total carbamazepine in SNEDDS was estimated by mixing 0.2 ml of the preparation with 1 ml of 1:1 ratio of methanol and chloroform followed by vortexing (Cyclomixer, Remi motors, Mumbai, India). The lysed preparation was then diluted with sufficient quantity of methanol and estimated in UV spectrophotometer. The drug content was obtained from the standard graph and average of three readings was taken for each preparation.
Release studies
The composition and method of preparation of various biorelevant media used in the study were followed according to United states pharmacopoeia national formulary (USP NF) – 2002. Before performing the release studies, standard graphs were plotted to calculate the CBZ content from the release study. in 0.1N HCl, Phosphate buffer, simulated gastric (SGF), simulated fasting gastric fluid (FASGF), simulated intestinal fluid (SIF), simulated fasting intestinal fluid (FASIF), simulated fed intestinal fluid (FESIF) to obtain the unknown concentration. The release of CBZ from SEDDS was determined using the membrane diffusion technique. Release studies of CBZ SNEDDS, CBZ SMEDDS and Tegretol suspension were run in triplicate at 37±0.5 ̊C using the USP II dissolution apparatus (Electrolabs, Mumbai) at 100 rpm. The in-vitro dissolution study is the major aspect of evaluation. One can predict the in-vivo absorption behavior of nanodispersions based on the release profiles of CBZ in various biorelevant media. Our aim of the study was to find out if there is an improved and improvised release pattern of carbamazepine from the SNEDDS dispersion when compared to the Tegretol suspension which is a marketed. So, the dissolution study of optimized dispersions and the marketed preparation (Tegretol suspension 100mg/5ml, Novartis – Mumbai) were carried out in all the simulated biorelevant media. The composition and method of preparation of all the biorelevant media were followed accordingly from USP/NF 2002. At the beginning of each experiment, size 0 hard gelatin capsules were filled with SNEDDS (cationic, anionic and nonionic) and SMEDDS. The capsule was then placed in 900 ml of respective biorelevant media (0.1N HCl, phosphate buffer, SGF, SIF, and FESIF). Capsules were held at the bottom of the vessel using stainless-steel sinkers. Initially, the dissolution medium was scanned spectrophotometrically from 900 to 200 nm at a gradient rate of 1.5–5 scans/min (Thomson UV spectrophotometer, UK). At the end of each experiment, the dissolution medium was visually examined for signs of turbidity or sedimentation and was judged as transparent, translucent, turbid, or milky. In the fasted state (FASGF, FASIF) resting volumes are quite low, and have been estimated to be about 25 ml [17, 18]. However, when a dosage form is administered, some fluid is usually co-administered. In pharmacokinetic studies, this volume is often in the 200–250 ml range. Assuming secretions at a rate of just under 1 ml/min [19], about 50 ml secretions are expected within 1 h, the longest period during which a fast disintegrating immediate release dosage form is expected to be totally emptied from the fasted stomach [20]. Thus, a realistic volume to simulate the total fluid available in stomach to dissolve simple dosage forms during gastric residence that empty with the fluid after administration in the fasted state would fall in the range of 250–300 ml. It is of note that in the USP dissolution apparatus II, often used for immediate release dosage forms, the minimum volume that can be used is slightly more than 300 ml. Otherwise, the paddle is not completely immersed in the dissolution medium. Based on the above theory, the volume of FASGF and FASIF dissolution mediums in the current studies were set at 500 ml. Samples of 5ml were withdrawn at preset time intervals. Immediately after the sample removal, the contents were replenished with 5ml of fresh medium. CBZ content in such samples was analysed UV spectrophotometrically at λmax 276nm. The calibration plots previously made in various media were used to estimate CBZ content in the samples.
Statistics
The data from different formulations was compared for statistical significance by using a non-parametric Kruskal – Wallis one-way analysis of variance (ANOVA) at a level of significance P<0.05 for the calculated parameters. All results were expressed as mean±SD.
Stability Studies
The stability of carbamazepine loaded SEDDS was assessed under various storage conditions namely room temperature, 30±2°C/65±5% RH and 40±2°C/75±5% RH as per ICH guidelines. SEDDS equivalent to 100 mg of CBZ was filled in size ‘000’ hard gelatin capsules. Ten such capsules were packed in Alu–Alu strip packs and stored at various aforementioned storage conditions up to 3 months. Samples were removed at 0, 30, 60 and 90 days and checked for physical appearance and content.
Results and Discussion
Solubility Studies
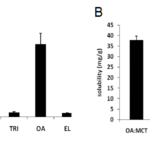
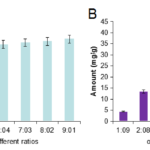
From the solubility study, it was found that the maximum solubility was observed in oleic acid followed by MCT and SBO. Solubility studies were performed to identify suitable oil, surfactant and cosurfactant that possess good solubilizing capacity for carbamazepine. Its solubility was high in oleic acid followed by MCT and SBO (Fig. 1A). Among the combinations of oleic acid with oils, OA:SBO and OA:MCT showed high solubility than other combinations (Fig. 1B). Accordingly, OA:SBO and OA:MCT were varied respectively. The ratios effect the solubility and it is evident that with 9:1 ratio in both the combinations exhibited the highest solubility of CBZ (Fig. 2A & B). Identifying the suitable oil, surfactant/cosurfactant having maximal solubilizing potential for drug under investigation is very important to achieve optimum loading and also to minimize the final volume of SEDDS.
Figure 1. Carbamazepine solubility in Individual oils (A) and Oil Combinations (B).
Figure 2. Carbamazepine solubility in various proportions of oleic acid (A) and MCT, Oleic acid and SBO (B).
The solubility effect of oleic acid in oils could be possibly due to micellar formation in such systems and subsequent solubility of CBZ into them. Oleic acid which is an unsaturated fatty acid is known to easily form a micelle in the gastrointestinal tract (Fig. 3).
Figure 3. Micellar solubilisation of carbamazepine
The dramatic difference in the solubility can be attributed to the chemical nature and HLB of the oily phases. Oleic acid has a HLB value of 1 and has surfactant like properties and is well known for its good solubilization potential. It is usually used as oily carrier for soft or hard gelatin capsule formulations. The blending of oleic acid with SBO and MCT mainly helps in improving the emulsification ability and this approach enhances bioavailability. Oleic acid was chosen due to its solubilizing potential for carbamazepine and also due to its long unsaturated chain fatty acid backbone which might help in improving lymphatic transport of thereby increasing its bioavailability.
Size, Zeta potential and Encapsulation
It is evident that the size, zeta potential and encapsulation from Fig. 4 , the size of SMEDDS dispersion ranged from 231- 670nm (Fig. 4A) with negative zeta potential ranging from 25-59mv (Fig. 4B). The percent encapsulated drug ranged from 60-75% (Fig. 4C) depending on the size of the dispersion. The overall stability in all the biorelevant release media ranged from 10-12hrs. Unlike SMEDDS, the size of cationic SNEDDS was small which ranged from 59-130nm with positive zeta potential of 12-21mv. The percent entrapment was between 77-89% . Similarly, the size of anionic SNEDDS was between 66.5-152nm with a negative zeta potential of 19-29mv. The percent encapsulation was comparatively less than that of cationic SNEDDS having 71-83%. The stability duration remained same as that of cationic SNEDDS. The size of nonionic SNEDDS was between 95-155nm with a zetapotential 0.57-2.36mv. The percent encapsulation which ranged from 76-82% .
Figure 4. Characterization of SEDDS. (A) Size of SEDDS in various biorelevant media. (B) Zetapotential of SEDDS in various biorelevant media. (C) Encapsulation of CBZ in SEDDS in various biorelevant media. All values are mean ±SD (n=3).
The size of cationic SNEDDS was small (108nm) in FASGF. This could be attributed to several possible reasons. One possibility can be due to the presence of the physiological surfactants like bile salts and lecithin, could have reduced the size because of their interfacial reducing properties as proposed by [21]. The second reason could be related to the fact that, CPC being a cationic surfactant and DOTAP a cationic lipid might be resisting the change in size in this specific pH of the media. The third hypothesis is that, it might be due to the partial inhibition of the lipase activity by the hydrophobic surfactant lecithin, which is supported by a research study which reports that, surfactants located at the oil droplet surface, can interfere with the attachment of the lipase complex to the oil water interface as stated by [22]. The drug solubilised in the oil droplets can interact with the surfactant chains and/or reduce the availability of the oil phase at the interface, thereby also interfering with the digestion process. Lecithin being an osmotically nonsensitive hydrophobic surfactant could also contribute to the stability and size reduction of the dispersion. Our findings correlated with a research study stating that halofantrine and procubol loaded SEDDS consisting of Cremophor RH40, sesame oil and ethanol were developed and evaluated in simulating fasted and fed state intestinal fluid with the resultant size reduction effect. SNEDDS could form stable nanoemulsions in all the release media. The mean size of the droplets was significantly not affected by any of the dispersion media and is supported by a research report with similar findings of size in various biorelevant media [23]. On the other hand, in 0.1N Hcl and phosphate buffer, the size of cationic SNEDDS dispersion ranges between 125 – 135nm which clearly indicates the absence of physiological surfactants and the effect of pH has enabled to increase the size in these media. The turbidity proportionately changed with size i.e; with increasing size the turbidity also enhanced.
The SMEDDS dispersions were achieved only for 12hrs in the absence of lecithin. Our findings imply that for a reasonable oil to surfactant ratio (≥1:1), a surfactant with HLB value greater than 10 would be necessary for fine and uniform self emulsification. It should be noted that head group structure, in addition to HLB, may play a role in the emulsification process [24]. Regression analysis yielded good predictive models for mean droplet size for both formulations with drug (p<0.0005, adjusted R2=0.444) and without drug (p<0.0005, adjusted R2=0.709). A higher oil-to-surfactant ratio resulted in a larger mean droplet size, with post-hoc pair wise comparisons indicating that mean droplet sizes from the 9:1 oil to surfactant ratio systems are significantly larger than those from the 1:1 ratio systems (p=0.005). Conversely, medium chain triglyceride, both of which have alkyl chain length of C8-C10 showed similar effects on droplet size. The phenomenon occurring might be low levels of partitioning of the surfactant at the oil water interface as a result of the low driving force, which would be the difference in carbon chain lengths between surfactant and oil. It has been proposed that oil with carbon chain length similar to that of surfactant penetrates more into the interfacial film, decreasing surfactant partitioning and droplet stabilization [25]. This relationship agrees with the results of James-Smith et al. [26], who correlated carbon chain length with mean emulsion diameter. Carbamazepine is thus relatively hydrophobic, but may still partition between the oil and aqueous layer along with the surfactant due to limited solubility in oil. Therefore a decrease in interfacial tension might occur that leads to an overall decrease in droplet size. Another potential reason for the change in droplet size may be the change in surfactant critical micelle concentration (CMC) due to addition of drug, which can increase surfactant aggregation number and decrease CMC [27]. At the same surfactant concentrations, systems with lower CMC values tend to have smaller droplet size [28]. The opposite effect of drug, however, has been observed for some systems. This decrease in zeta potential magnitude can be correlated with the comparatively larger droplet sizes as well as the increased drug concentration of these same systems. Surface charge of the droplets was not statistically related to any of the formulation parameters.
The pH and the surfactants might be the determining factors for the size change. Potassium docusate being a highly ionizable anionic surfactant could have pH dependent surfactant properties. In contrary, in the remaining media, the size was comparatively large. However, the size of non-ionic SNEDDS was high in all the release media. This could be possibly due to the absence of charged surfactants thus a decrease in the repulsive forces between the oil droplets.
Release studies
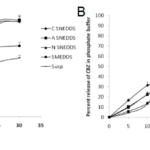
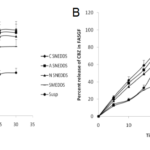
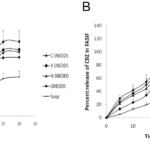
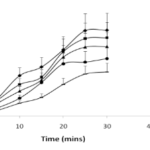
The release of carbamazepine from SNEDDS and SMEDDS was compared with Tegretol suspension. An initial rapid release (burst effect) was seen in cationic SNEDDS and further it gradually increased to 79.45% at the end of 15mins. Though the initial release rate of anionic SNEDDS was lower than cationic SNEDDS, after 20mins, it’s rate however increased. The total percent release of all the SNEDDS (cationic, anionic and nonionic) dispersions ranged from 75-95% in 30 mins. The total percent release of SMEDDS and suspension were 59.07% and 49.03% respectively in 30 mins. Overall, a poor release of carbamazepine was observed from suspension in 30 mins (Fig. 5A). In comparision to release in 0.1N HCl, in phosphate buffer, the release of carbamazepine from cationic SNEDDS, SMEDDS and suspension had been drastically increased but no significant change was seen with anionic and nonionic SNEDDS dispersions (Fig. 5B). A similar pattern of release was observed in SGF. The release of carbamazepine from all the SNEDDS remained fairly same (77% – 83%) but an increase was seen in SMEDDS compared to suspension (Fig. 6A). In FASGF the extent of release of carbamazepine from cationic SNEDDS was increased to 94% followed by anionic SNEDDS i.e, 89.96%. A significant difference was not seen in the remaining dispersions (Fig. 6B). Surprisingly in SIF and FASIF, the release of carbamazepine from nonionic SNEDDS, SMEDDS and suspension were almost same. The release from cationic SNEDDS was highest in FASIF i.e; 97.39% and only 86.34% was seen in SIF (Fig. 7A and Fig. 7B). In FESIF, only 49% release of carbamazepine was seen in suspension but 63% from SMEDDS.The release from cationic SNEDDS was same in FASGF and FESIF followed by anionic and nonionic SNEDDS (Fig. 8).
Figure 5. Release profiles of CBZ in (A) 0.1N HCl and (B) Phosphate Buffer.
Figure 6. Release profiles of CBZ in (A)SGF and (B) FASGF.
Figure 7. Release profiles of CBZ in (A)SIF and (B) FASIF.
Figure 8. Release profiles of CBZ in FESIF
The exponential relationship between turbidity and release of CBZ implies that turbidity might be a beneficial parameter with regard to size and release when developing SNEDDS formulations based on in vitro characterization data. A research report states that, release profiles constructed in simulated physiological dissolution conditions may be crucial for the assessment of the absorption profile of lipophilic drugs [29]. This shows the importance of lipophilic surfactant (lecithin) in achieving invivo simulated release patterns of the SNEDDS [30] dispersions to get predictable absorptions patterns.
Stability Studies
No change in the physical parameters such as homogeneity and clarity was observed during the stability studies. No decline in the carbamazepine content was observed at the end of 3 months indicating that carbamazepine remained chemically stable in SEDDS. Furthermore, no change in release efficiency was observed for the carbamazepine loaded SEDDS.
Optimum levels of oil, surfactant, and cosurfactants are necessary to produce a thermodynamically stable nanoemulsion system. Besides, SEDDS formulations are generally put into hard gelatin capsules as the final dosage form. These two factors play the pivotal role in maintaining the ultimate stability of the final SEDDS dosage form. Liquid-filled hard gelatin capsules are susceptible to leakage, and the entire system has a very limited shelf-life, owing to its liquid characteristics and the possibility of precipitation of the drug from the system.
Due to these reasons, the developed formulations were subjected to accelerated stability testing (40°C/75% relative humidity) to evaluate their stability and the integrity of the final dosage form as well. Physical characteristics (color, visual clarity, phase separation/ precipitation) were re-evaluated after one, two and three months of formulation-time. Precipitation of CBZ was not seen during the entire release study which indicates that digestion of oils is totally absent.
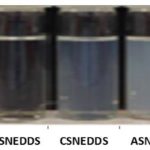
In the stability studies, the turbidity was also assessed (Fig. 9). The turbidity showed a good linear correlation between them. The BCSNEDDS, BASNEDDS and BNSNEDDS which do not contain oil were transparent. The SNEDDS (cationic, anionic and non-ionic) resulted in nanoemulsion showing hazy blue appearance with an average size less than 200nm respectively while the SMEDDS was judged as turbid or milky which resulted in the size range above 200nm (macroemulsion). As reported in the literature, the visual appearance of the dispersion is a measure of the spontaneity of emulsification and an indirect measure of the globule size of the dispersed phase in the self-emulsifying system [31]. Measurement of turbidity of the medium during the dispersion process was recently used as an alternative method to quantify the emulsification rate of SEDDS formulations and provide rapid real time estimate of droplet size. The turbidity of the dispersion during the size analysis was measured at 420 nm. Measured turbidity supported the visual examination (i.e. the transparent to hazy blue SEDDS gave smaller absorbance and the milky white SEDDS gave maximum absorbance). During the emulsification process, the measured turbidity and scattered light relative intensity were correlated with time. SNEDDS appeared transparent to hazy blue appearance which showed lower droplet size. These results are in agreement with those obtained and established a comparison between visual observation and droplet size. The SMEDDS and SNEDDS at different compositions impart different visual properties when dispersed in aqueous media. Such observations are frequently used to optimize SEDDS formulations. As quoted in the stability studies about the interfacial adsorption of physiological surfactants and resultant size [32] reduction, this was true in the release studies. The total percent release from cationic SNEDDS is near to 95% in FASGF, FASIF and FESIF which holds good to the theory stating that, lesser the size, higher the encapsulation [33] more the rapid and extent of release. The same phenomena could be seen in all the biorelevant media. In the remaining media, only 70-80% of CBZ released was observed.
Figure 9. Visual Appearance of carbamazepine SEDDS preparations. BCSNEDDS: Blank Cationic Self Nano Emulsifying Drug Delivery System; BASNEDDS: Blank Anionic Self Nano Emulsifying Drug Delivery System; BNSNEDDS: Blank Nonionic Self Nano Emulsifying Drug Delivery System; CSNEDDS: Cationic Self Nano Emulsifying Drug Delivery System; NSNEDDS: Nonionic Self Nano Emulsifying Drug Delivery System; ASNEDDS: Anionic Self Nano Emulsifying Drug Delivery System; SMEDDS: Self Micro Emulsifying Drug Delivery System
similar trend of rapid and higher extent of release of CBZ from anionic SNEDDS dispersion was observed. But such phenomena could not be seen for non-ionic SNEDDS. The total percent release [34] was restricted to 75% which was almost similar in all the biorelevant media. The hypothesis of size and surfactant effect could not be applied. One possibility could be due the absence of charged surfactants and the change in orientation.
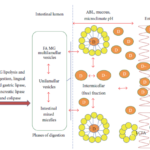
The primary mechanism of action by which SEDDS formulation leads to improved bioavailability with rapid release of carbamazepine is usually avoidance of the slow dissolution process which limits the bioavailability from solid dosage forms. Preferably, the formulation allows the drug to remain in a dissolved state throughout its transit in the GIT. It is evident from Figure 10 that SNEDDS enhances [35] release of carbamazepine and thus oral bioavailability enabling reduction in dose and more consistent temporal profiles of absorption [36, 37]. Thus, self-emulsifying formulations are readily dispersed in the GI tract, where the motility of the stomach and small intestine provides the agitation necessary for emulsification. The potential advantages of the self-emulsifying systems include no dissolution step required, solubility enhancement and rapid release, increase in rate and extent of absorption and thus increased bioavailability. SEDDS deliver BCS Class II drugs like carbamazepine effectively. Further, they provide consistent temporal profile with reduced dosing and dosing frequency. No specific relationship could be observed in the release pattern from SMEDDS. Because of high variability in size with time in all the release media, the drug diffusion through the droplet could be non-uniform and unpredictable. With increase in size, the rate and total percent release was declined. In conclusion, the physiological surfactants, hydrophobic & hydrophilic surfactants and pH could be the determining factors to determine the actual size of SNEDDS and SMEDDS that ultimately effects the overall release of the drug thus effecting absorption subsequently.
Figure 10. Schematic diagram of intestinal drug release and transport from SEDDS formulations via the portal and the mesenteric lymphatic routes. (A) Increased membrane fluidity facilitating transcellular absorption. (B) Opening of tight junctions to allow paracellular transport. (C) Inhibition of P-gp and/or CYP450 to increase intracellular concentration and residence time. (D) Stimulation of lipoprotein/chylomicron production. ABL: aqueous boundary layer; D: Carbamazepine; FA: fatty acid; LCFA: long chain fatty acid; ME: microemulsion; MG: monoglyceride; SEDDS: self-emulsifying drug delivery system; TG, triglyceride; TJ, tight junction.
Conclusions
The study evaluates comparison of size, zetapotential and release data to demonstrate the different roles played by SMEDDS, SNEDDS and suspension of carbamazepine. The extent of solubilization is directly related to the composition of the SEDDS formulation. The individual ingredients of SEDDS formulation often found significant and in times unique role in release of carbamazepine. The oil phase, exemplified by oleic acid is an essential component of SEDDS formulation and is required for the solubilization and/or emulsification of carbamazepine. Therefore, optimizing the ratio of primary and secondary surfactants, and oil are essential to produce SEDDS with desirable in vitro characteristics. In this study, however, no correlation was observed between chared and uncharged SEDDS and between the extent of release and encapsulation. Based on these data it could be concluded that standard dissolution studies are suitable for optimizing SEDDS formulations and for identifying critical formulation variables. While a weak correlation was observed between SMEDDS and suspension release studies are better suited to predict the performance of carbamazepine in biorelevant media. The CSNEDDS was found to be the best system in terms of size and total release of carbamazepine. In conclusion, the rate and extent of release of carbamazepine from cationic SNEDDS was more in all the biorelevant media. Only 59-69% release was observed in SMEDDS and a variable increase i.e, 42-62% from suspension. A prominent feature seen in nonionic SNEDDS is that, only 75-80% was consistently released in all the biorelevant media. Next to cationic SNEDDS, the release of carbamazepine was observed in anionic SNEDDS followed by nonionic SNEDDS.
Competing interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors are grateful to Natco Research Center (NRC), Sanathnagar, Hyderabad for providing financial support of the work and is sincerely acknowledged. We are grateful to the entire NDDS unit team of NRC for their assistance in the analytical measurements and their efforts are greatly appreciated.
References
- Sznitowska M., Janicki S., Dabrowska E., Zurowska Pryczkowska K. Submicron emulsions as drug carriers: studies on destabilization potential of various drugs, Eur. J. Pharm. Sci. 2001;12:175-179.
- Becirevic Lacan M., Jug M., Bacic Vrca V., Cetina Cizmek B. Development of o/w emulsion formulation for carbamazepine by using modified cyclodextrins, Acta Pharm. 2002; 52:149-159.
- Akkar A., Muller R.H., Formulation of intravenous carbamazepine emulsions by SolEmuls® technology, Eur. J. Pharm. Biopharm. 2003;55:305-312.
- Constantinides P.P., Tustian A., Kessler D.R. Tocol emulsions for drug solubilization and parenteral delivery, Adv. Drug Deliv. Rev. 2004;56: 1243-1255.
- D. Yang, J. Zhu, Y. Zheng, and L. Ge. Preparation, characterization and pharmacokinetics of sterically stabilized nimodipinecontaining liposomes. Drug Dev. Ind. Pharm. 32:219–227 (2006). Washington C.
- R. N. Gursoy, S. Benita. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs.Biomed. Pharmacother. 58:173–182 (2004).
- Floyd A.G. Top ten considerations in the development of parenteral emulsions Pharm. Sci. Technol. 1999; 2:134-143.
- Pouton C.W. Lipid formulations for oral administration of drugs: Nonemulsifying Self-emulsifying and ‘self-microemulsifying’ drug delivery systems Eur. J. Pharm. Sci. 2000; S93-S98.
- Sperling M.R. The consequences of uncontrolled epilepsy, CNS Spectr. 2004; 9:98-9.
- Genaro A.R. Remington The Pharmaceutical Sciences and Practice of Pharmacy Lippincott, Williams & Wilkins, Philadelphia.
- Goodman L.S., Gilman A.G., Hardman J.G., Limbird L.E. Goodman & Gilman’s the pharmacological basis of therapeutics McGraw Hill Book Co, New York. 2001.
- Sweetman S.C. Martindale. The Complete Drug Reference. Pharmaceut-ical Press, London UK. 2006.
- Lund W. The Pharmaceutical Codex: Principles and Practices of Pharmaceutics 12th ed. The Pharmaceutical Press, London. 1994.
- Kobayashi Y., Itai S., Yamamoto K. Physicochemical properties and bioavailability of carbamazepine polymorphs and dehydrate, Int. J. Pharm. 2000; 193:137-146.
- Sethia S., Squillante E. Physicochemical characterization of solid dispersions of carbamazepine formulated by supercritical carbon dioxide and conventional solvent evaporation method J. Pharm Sci. 2002;91:1948-1957.
- Vitale S.A., Katz J.L. Liquid droplet dispersions formed by homogeneous liquid-liquid nucleation: “The Ouzo effect”, Langmuir. 2003; 19:4105- 4110.
- Bouchemal K., Briancon S., Perrier E., Fessi H. Nanoemulsion formulation using spontaneous emulsification:solvent, oil and surfactant optimization, Int J. pharm. 2004;280:241-251.
- Maltby J.R., Sutherland A.D., Sale J.P., Shaffer E.A. Preoperative oral fluids:Is a five hour fast justified prior to elective surgery? Anesth. Analg. 1986; 65:1112-1116.
- Lydon A., Murray C., McGinley J., Plant R., Duggan F., Shorten G. Cisapride does not alter gastric volume or pH in patients undergoing ambulatory surgery Can J. Anaesth., 1999; 46:1181-1184.
- Dubois A., Eerdewegh P.V., Gardner J.D. Gastric emptying and secretion in Zollinger Ellison syndrome J.Clin. Invest. 1977; 59:255-263.
- Oberle R.L., Chen T.S., Lloyd C., Barnett J.L., Owyang C., Meyer J., Amidon G.L. The influence of the interdigestive migrating myoelectric complex on the gastric emptying of liquids Gastroenterology. 1990; 99:1275-1282.
- Goddeeris C, Coacci J., Van den Mooter G. Correlation between digestion of the lipid phase of smedds and release of the anti-HIV drug UC 781 and the antimycotic drug enilconazole from smedds European Journal of Pharmaceutics and Biopharmaceutics, 2007;66:173-181.
- Embleton Jonathan K., Pouton Colin W. Structure and function of gastrointestinal lipases Advanced Drug Delivery Reviews. 1997; 25:15-32.
- James Smith MA., Alford K., Shah D.O., A novel method to quantify the amount of surfactant at the oil/water interface and to determine total interfacial area of emulsions, J. Colloid Interface Sci, 2007; 310(2):590–598.
- Patist A, Chhabra V, Pagidipati R, Shah R, Shah D.O, Effect of chain length compatibility on micellar stability in sodium dodecyl sulfate/alkyltrimethylammonium bromide solutions, Langmuir, 1997; 13(3): 432–434.
- James Smith MA, Alford K, Shah DO, A novel method to quantify the amount of surfactant at the oil/water interface and to determine total interfacial area of emulsions, J. Colloid Interface Sci, 2007; 310(2):590–598.
- Chiu YC, Han YC, Cheng HM, Relationship of solubilization rate to micellar properties. Anionic and nonionic surfactants, ACS Symp. Ser. 253(Struct./Perform. Relat. Surfactants), 1984:89–105.
- Goloub TP, Pugh RJ, The role of the surfactant head group in the emulsification process: binary (nonionic-ionic) surfactant mixtures, J. Colloid Interface Sci, 2005; 291(1): 256–262.
- Flemming N., Emilie Gibault., Helena Ljusberg Wahren., Lise Arleth., Jan Skov Pedersen., Anette mullertz. Characterization of Prototype Self Nanoemulsifying Formulations of Lipophilic Compounds Journal of Pharmaceutical Sciences. 2007; 96(4):876-892.
- Craig D.Q.M., Lievens H.S.R., Pitt K.G., Storey D.E. An investigation into the physico-chemical properties of self-emulsifying systems using low frequency dielectric spectroscopy surface tension measurements and particle size analysis. Int J.Pharm. 1993; 96:147-155.
- Sami Nazzal, Mansoor Khan A. Response Surface Methodology for the optimization of Ubiquinone Self-Nanoemulsified Drug Delivery System AAPS Pharm Sci Tech. 2002;3 (1):1-9.
- Vertzoni M., Dressman J., Butler J., Hempenstall J., Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm. 2005;60(3):413-7.
- Agarwal R. Katare O.P., Vyas S.P. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol Int J.Pharm. 2001;228:43-52.
- Arunothayanun P., Bernard M.S., Craig D.Q.M., Uchegbu I.F., Florence A.T. The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether Int J Pharm. 2000; 201:7–14.
- Ruckmani K., Jayakar B., Ghosal S.K. Nonionic surfactant vesicles (niosomes) of cytarabine hydrochloride for effective treatment of leukemia encapsulation storage and in vitro release Drug Dev Ind Pharm. 2000;26:217–222.
- Glavas-Dodov M., Goracinova K., ladenovska K.M and Fredro- Kumbaradzi E. Release profile of lidocaine HCl from topical liposomal gel formulation. Int J. Pharm. 2002; 242:381-384.
- O’Driscoll C. M. Lipid-based formulations for intestinal lymphatic delivery. European Journal of Pharmaceutical Sciences. 2002; 15(5):405–415.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/