J Med Discov (2017); 2(2):jmd17013; DOI:10.24262/jmd.2.2.17013; Received March 31st,2017, Revised April 15th, 2017, Accepted April 20th, 2017, Published May 5th, 2017.
Fusion proteins CDGM and 4CDGM enhance B cell proliferation
Zehua Sun1, Xiaoyi Lu2, Shiqiang Lu3,*
1Department of Medicine, National Jewish Health, 1400 Jackson Street, Denver, CO, 80206, USA
2Department of Philosophy, Faculty of Arts, the University of Hong Kong, Pokfulam, 999077, Hong Kong
3Genscript USA Inc. 860 Centennial Ave. Piscataway, NJ 08854, USA
* Correspondence: Shuqiang Lu, Genscript USA Inc. 860 Centennial Ave. Piscataway, NJ 08854, USA. Email: shiqiang.lu@genscript.com.
All the authors contributed equally to this work.
Abstract
Epstein-Barr Virus (EBV) induced B cell immortalization is an efficient tool for monoclonal antibody screening and isolation. However, the low efficiency of B cell transformation by EBV limits the usage of this technique. Numerous cytokines are reported to activate and boost B cells proliferation. CD40L are also reported to provide signals to B cells that induce proliferation. In order to answer that whether cytokines and CD40L fusion protein can enhance the EBV transformation efficiency of B cells, two fusion proteins CDGM (CD40L-GM-CSF) and 4CDGM (IL-4-CD40L-GM-CSF) are designed and expression plasmids are constructed. Our results showed that both CDGM and 4CDGM can enhance B cell proliferation with higher efficiency to increase B cell proliferation in vitro. However, CDGM and 4CDGM will reduce the proliferation of B cells in the presentation of EBV infection. Both CDGM and 4CDGM could facilitate the proliferation of monocytes. Our results provide indication in B cell proliferation and optimization of EBV induced B cell immortalization.
Keywords: Epstein-Barr Virus, B cell immortalization, CD40L-GM-CSF, IL-4-CD40L-GM-CSF, fusion protein
Introduction
The Epstein-Barr Virus (EBV) induced B cells immortalization is a powerful tool for antibody screening and isolation, but is limited in application due to the low transformation efficiency [1-5]. Increased B cell immortalization efficiency is reported after EBV transformation in the presence of CD40L-expressing feeder cells. CD40L was identified as the most important costimulatory molecule for B cell activation and proliferation, so it was not surprising to find that CD40L-expressing fibroblast could be used as feeder cells and induce the rapid expansion of human B cells in vitro [6-9]. This CD40L-B system has been used for short-term culture of human PBMCs to obtain highly purified B cells, which showed great therapeutic value for cancers and infectious diseases [10]. Cytokines are also observed in roles of B cell proliferation[11-13]. Numerous cytokines have been documented to activate and boost B cell proliferation. Certain cytokines can synergistically enhance the proliferation of B cells after EBV transformation [14-16]. However, whether the CD40L and cytokines can be used synergistically to enhance EBV transformation remains unknown.
GM-CSF and IL-4 are two cytokines that commonly used in B cell activation and proliferation [17-20]. In order to study whether CD40L and cytokines can be used synergistically in enhancing the efficiency of EBV-induced B cell transformation, two fusion proteins were constructed. CD40L was fused with cytokine GM-CSF and IL-4 respectively. Our results showed that both CDGM and 4CDGM can enhance B cell proliferation with higher efficiency. However, CDGM and 4CDGM will reduce the B cell transformation efficiency in the presence of EBV infection. Both CDGM and 4CDGM can facilitate the proliferation of monocytes. Our results provide indication that EBV induced B cell proliferation and immortalization.
Materials and Methods
Growth medium for cell culture
Dulbecco’s Modified Eagle Medium (DMEM) growth medium for 293T: DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/ml penicillin, and 100 µg/ml streptomycin. Growth medium for 293F cells: FreeStyle™ 293 Expression Medium. Growth medium for EBV transformation: Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% FBS, 0.66 µg/ml cyclosporin A (CsA), 100 units/ml penicillin, 100 µg/ml streptomycin and 50μM β-mercaptoethanol. Growth medium for CD40L-B cells: IMDM supplemented with 10% FBS, 0.66 µg/ml CsA, 2 µg/ml IL-4, 5 µg/ml insulin, 30 µg/ml transferrin, 100 units/ml penicillin, 100 µg/ml streptomycin and 50μM β-mercaptoethanol.
pSecTag2B plasmid was used for the expression of fusion cytokines, CDGM and 4CDGM. pSecTag2B expression vector purchased from Life Technologies was about 5.2kb, which was designed for high-level expression of target proteins in a stable or transient way. Given that the expression of target protein was initiated by T7 promoter, the presence of vaccinia virus vTF7.3 could increase its expression to a much higher level. The vTF7.3 virus was brought by Dr. Meiyun Zhang from the National Institute of Health (NIH), USA.
Flow cytometry analyses were performed on FACScalibur (BD Sciences) [24]. For phenotyping, cells were stained with anti- CD3, CD19 antibodies at 4 °C for 30min before analysis. Data were analyzed by FlowJo software (Treestar, Ashland, OR).
Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) is a commonly used assay to evaluate cell proliferation of lymphocytes in vitro and in vivo by labeling with dye dilution to track cell multiple generations by flow cytometry. The optimal concentration of CFSE for B cell staining was 5 μM as determined by tracking five cell generations. The CFSE manual for labeling cells in suspension was strictly followed. In brief, B cells were incubated with 5 μM CFSE at room temperature for 20 min without light. Cells were washed five times in the original staining volume of growth medium before treatmentwith different stimulators. Finally, cells proliferation was examined by flow cytometry for FITC signal on day 6.
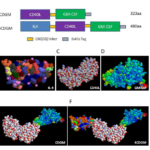
Figure 1. Predicted 3D structure of fusion cytokines. (A) Schematic of CDGM and 4CDGM. (B–D) Structure of IL4 (B), CD40L (C), and GM-CSF (D) were obtained from the RCSB Protein Data Bank (http://www.rcsb.org/pdb/home). (E) Predicted 3D structure of fusion protein CDGM. (F) Predicted 3D structure of 4CDGM.
Results
Design of fusion proteins CDGM and 4CDGM
In order to investigate whether CD40L co-expressed with GM-CSF or IL4 as fusion protein can better stimulate human B cell proliferation and immortalization, two fusion proteins were designed accordingly (Fig. 1). One fusion protein, termed CDGM, was constructed by coupling human CD40L with human GM-CSF (Asp108-Leu261, deletion of transmembrane domain) via10aa [GS] ×linker. The other fusion protein, termed 4CDGM, was constructed by adding human IL-4 in the N-terminal of CDGM with the same linker. The amino acid sequences of CDGM and 4CDGM are shown in Figure 2. The 3D structure of IL4 (Fig. 1B), CD40L (Fig. 1C) and GM-CSF (Figure 1D) were obtained from the RCSB Protein Data Bank (http://www.rcsb.org/pdb/home). The predicted 3D structure of CDGM (Fig. 1E) and 4CDGM (Fig. 1F) were produced by RASWIN software based on PDB information of IL4, CD40L and GM-CSF.
Figure 2. Full amino acid sequences of CDGM and 4CDGM. The 10× [GS] linker is in brown letters as GSGSGSGSGS. The 6×His tag is in gray letters as HHHHHH. The Myc epitope is in green letters as ARGGPEQKLISEEDLNSAVD. The signal peptide is underlined in the N-terminal of both fusion cytokines.
Expression and purification of CDGM and 4CDGM
PBMCs were used as a template for gene cloning due to the high expression level of IL-4, CD40L, and GM-CSF in activated T cells and macrophages. PBMCs were isolated from buffy coats by density gradient centrifugation. RNA was then extracted and cDNA was synthesized. The genes of IL-4, CD40L, GM-CSF, and BAFF are specifically amplified using cDNA as template (Fig. 3A). The primers (Tab. 1) containing linker gene and 6×His tag were designed for SOE-PCR to generate recombinant fusion genes (Fig. 3B). The recombinant genes (CDGM and 4CDGM) were then inserted into mammalian cell expression vectors. The sequences of fusion genes were confirmed by DNA sequencing. After verification the sequence, we tested whether recombinant proteins can be expressed and secreted into the supernatant. 293T cells were transfected with pCDGM or p4CDGM, and incubated for 2 days at 37 °C and 5%CO2. The supernatants were harvested and analyzed by western blot. The sizes of CDGM and 4CDGM were around 36 and 54 kDa, respectively. The expression of CDGM and 4CDGM was confirmed by anti-His antibodies. However, non-specific proteins existed for both samples. These two proteins were expressed by 293F cells in a 200 ml culture volume. After 4 days, the supernatants were harvested and purified by the Ni-NTA purification system. Purified CDGM and 4CDGM were at 0.81 and 1.27 mg/ml, respectively. Hence, fusion cytokines, CDGM and 4CDGM, could be readily expressed by mammalian cells and purified by the Ni-NTA system.
Figure 3. Cloning of fusion cytokines into mammalian cell expression vectors. (A) Amplification of the IL-4, CD40L, GM-CSF and BAFF genes from cDNA of human PBMCs. (B) Strategy for generating fusion genes of CDGM and 4CDGM by SOE-PCR. (C) CDGM and 4CDGM were cloned into mammalian cell expression vector.
| Purpose | name | sequence |
| PCDNA:huCD40L | CD40L-F | TAT ATC TAG AAT GAT CGA AAC ATA CAA CCA A |
| CD40L-R | TAT AGA ATT CTC AGA GTT TGA GTA AGC CAA | |
| p4CDGM | IL4-P1 | TATAGAATTCGCCACCATGGGTCTCACCTCCCAAC |
| IL4-P2 | TTAACTCGAGGCTGCCGCTACCGCTACCGCTGCCGCTACCGCTCGAACACTTTGAATATTTC | |
| CD40L2-P1 | TATACTCGAGAACAGCTTTGAAATGCAAAAAG | |
| CD40L2-linkerP2 | TTAATCTAGAGCTGCCGCTACCGCTACCGCTGCCGCTACCGAGTTTGAGTAAGCCAAAGG | |
| GMCSF3-P1 | TATATCTAGAATGTGGCTGCAGAGCCTGCTG | |
| GMCSF3-P2 | TTAAGGATCCTCATCAATGGTGGTGGTGATGATGCTCCTGGACTGGCTCCCAGCAG | |
| pCDGM | B2G2-For | TATAGGATCCAACAGCTTTGAAATGCAA |
| G2-Rev | TAATCTCGAGCCTCCTGGACTGGCTCCCA |
Table 1. Primers designed for gene cloning and sequencing
Recombinant fusion cytokines enhanced proliferation and activation of B cells in vitro
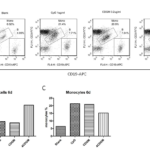
In order to address whether fusion proteins can induce the activation and proliferation of human B cells in vitro, freshly isolated PBMCs were incubated in the presence of PBS (blank control), CpG (postive control), CDGM and 4CDGM for 6 days. B cells were assessed by flow cytometry (Fig. 4A). Rapid proliferation of B cells was observed in the presece of CpG. CDGM showed a similar efficiency for the stimulation of B cells as CpG. Interestingly, 4CDGM was more efficient to induce the proliferation of B cells compared with CpG and CDGM (Fig. 4B). Moreover, CDGM and 4CDGM could enhance the proliferation of monocytes even in the presence of CsA, a calcineurin inhibitor that exerted immunosuppressive effects through the downregulation of nuclear factor of activated T cells (NFAT) transcription factor and prevented transcription activity of T cell effector cytokines (Fig. 4C). We determined whether CDGM and 4CDGM work synergitically with EBV for better immortalization efficiency. Intriguingly, the efficiencies of EBV transformation were much lower in the presence of CDGM and 4CDGM (<1%). In this experiment, PBMCs were used for EBV transfromation along with CsA. the rapid activation and expansion of T cells were suspected to be the main reasions for the reduction in B cell proliferation, because the activation and expansion of T cells could be readily observed within 2 days after the addition of CDGM and 4CDGM even in the presence of 0.66 µg/ml CsA, which was proved to be sufficient for inhibiting T cell proliferation in vitro.
Figure 4. Recombinant fusion cytokines enhanced proliferation and activation of B cells in vitro. (A) PBMC was treated with CpG, CDGM, and 4CDGM and then examined on day 6 by flow cytometry. (B) B cells in the whole population. (C) Monocytes in the whole population.
Discussion
EBV-induced B cell immortalization is a powerful tool for antibody screening and B cell culture. However, this technique is limited by the low B cell transformation efficiency. This has been demonstrated in details in our previous study [1, 21-23]. To minimize the detrimental effects caused by feeder cells and enlightened by the recent discovery that the fusion cytokine composed of IL-4 and GM-CSF can induced fast activation and proliferation of B cells, two fusion proteins were designed accordingly: CDGM was constructed by coupling transmembrane domain-deleted human CD40L (Asp108-Leu261) with human GM-CSF; 4CDGM was constructed by the addition of human IL-4 in the N-terminal of CDGM. Both fusion cytokines, especially 4CDGM, could significantly enhance the proliferation for primary human B cells. Intriguingly, when CDGM and 4CDGM were present with EBV infection, they reduced the proliferation of B cells. Both CDGM and 4CDGM could facilitate the proliferation of monocytes. These observations are needed further investigation to discover the potential mechanisms. Our results provide indications in EBV transformation in human B cells and optimization of EBV-induced B cell immortalization.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
We thank Dr. Meiyun Zhang from the National Institute of Health (NIH), USA to provide us with the vTF7.3 virus.
References
- Sun, Z., et al., Isolation and characterization of HIV-1 envelope glycoprotein specific B cell from immortalized human naive B cell library. J Gen Virol, 2017.
- Zhang, B., et al., The CD40/CD40L system: a new therapeutic target for disease. Immunol Lett, 2013. 153(1-2): p. 58-61.
- Chen, K., et al., CD40/CD40L dyad in the inflammatory and immune responses in the central nervous system. Cell Mol Immunol, 2006. 3(3): p. 163-9.
- Voorzanger-Rousselot, N. and J.Y. Blay, Coexpression of CD40 and CD40L on B lymphoma and carcinoma cells: an autocrine anti-apoptotic role. Leuk Lymphoma, 2004. 45(6): p. 1239-45.
- Briones, J., J. Timmerman, and R. Levy, In vivo antitumor effect of CD40L-transduced tumor cells as a vaccine for B-cell lymphoma. Cancer Res, 2002. 62(11): p. 3195-9.
- van Kooten, C. and J. Banchereau, CD40-CD40 ligand. J Leukoc Biol, 2000. 67(1): p. 2-17.
- DiSanto, J.P., et al., CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature, 1993. 361(6412): p. 541-3.
- Xu, J., et al., Mice deficient for the CD40 ligand. Immunity, 1994. 1(5): p. 423-31.
- Arpin, C., et al., Generation of memory B cells and plasma cells in vitro. Science, 1995. 268(5211): p. 720-2.
- Schultze, J.L., et al., CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest, 1997. 100(11): p. 2757-65.
- Kooy-Winkelaar, Y.M., et al., CD4 T-cell cytokines synergize to induce proliferation of malignant and nonmalignant innate intraepithelial lymphocytes. Proc Natl Acad Sci U S A, 2017. 114(6): p. E980-E989.
- Sprent, J., et al., T-cell proliferation in vivo and the role of cytokines. Philos Trans R Soc Lond B Biol Sci, 2000. 355(1395): p. 317-22.
- Armitage, R.J., et al., Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol, 1993. 150(9): p. 3671-80.
- Mordasini, V., et al., Activation of ATR-Chk1 pathway facilitates EBV-mediated transformation of primary tonsillar B-cells. Oncotarget, 2017. 8(4): p. 6461-6474.
- Sadreddini, S., et al., Evaluation of EBV transformation of human memory B-cells isolated by FACS and MACS techniques. J Immunotoxicol, 2016. 13(4): p. 490-7.
- Steinitz, M. and G. Klein, EBV-transformation of surface IgA-positive human lymphocytes. J Immunol, 1980. 125(1): p. 194-6.
- Deng, J., et al., Engineered Fusokine GIFT4 Licenses the Ability of B Cells to Trigger a Tumoricidal T-cell Response. Cancer Res, 2014. 74(15): p. 4133-44.
- Ni, K. and H.C. O’Neill, Proliferation of the BCL1 B cell lymphoma induced by IL-4 and IL-5 is dependent on IL-6 and GM-CSF. Immunol Cell Biol, 1992. 70 ( Pt 5): p. 315-22.
- Quandt, D., et al., Synergistic effects of IL-4 and TNFalpha on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation. J Transl Med, 2014. 12: p. 151.
- Morris, S.C., et al., Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J Immunol, 2009. 182(3): p. 1429-38.
- Lu, S., Z. Sun, and M.-y. Zhang, Generation of immortalized human naïve B cell libraries by optimized EBV transformation. Journal of Medical Discovery, 2016. 2(1).
- Yang, Z., et al., Identification of Non-HIV Immunogens That Bind to Germline b12 Predecessors and Prime for Elicitation of Cross-clade Neutralizing HIV-1 Antibodies. PLoS One, 2015. 10(5): p. e0126428.
- Liu, C., et al., An Optimized method for construction of Epstein-Barr virus-transformed immortalized lymphoblastoid cell lines. Journal of Medical Discovery, 2016. 2(1).
- Sun, Z., et al., Reconstitution and characterization of antibody repertoires of HIV-1-infected “elite neutralizers”. Antiviral Res, 2015. 118: p. 1-9.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/