J Med Discov (2017); 2(2):jmd17003; DOI:10.24262/jmd.2.2.17003; Received March 10th, 2017, Revised April 10th, 2017, Accepted April 14th, 2017, Published April 21st, 2017.
Dig the root of cancer: targeting cancer stem cells therapy
Jing Xiao1, Jiasheng Mu2,3, Tianrun Liu4, Haineng Xu5,*
1Shanghai Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200031, China
2Department of General Surgery, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China
3Institute of Biliary Tract Disease, Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China
4Department of Otorhinolaryngology – Head and Neck Surgery, The Sixth Hospital of Sun Yat-sen University, Guangzhou 510655, China
5Department of Radiation Oncology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, 19104
* Correspondence: Haineng Xu, 3400 Civic Blvd., Department of Radiation Oncology, University of Pennsylvania School of Medicine, Philadelphia, PA, 19104. Email: xuhaineng123@163.com.
Abstract
Cancer stem cells are a subpopulation of cancer cells which have stem cell characteristics and play a vital role in tumor formation, metastasis, relapse and resistance to chemo- drugs. Exploring drugs or designing novel approaches to target cancer stem cells will contribute greatly to cancer therapy. The first step of exploring effective drugs to cancer stem cells is to acquire cancer stem cells and characterize their properties. Developing the targeting drugs could be based on the characteristics of cancer stem cells, like signal pathways contributing to self-renew, the ability to pump small molecules out and quiescence. This review will summarize the current approaches to designing drugs to target cancer stem cells.
Keywords: cancer stem cells, self-renew, targeting therapy
Introduction
Cancer is a severe disease leading to millions of death in the worldwide [1, 2] and it is now first cause of death. The most used methods for cancer treatment are surgery, chemo-therapy and radio-therapy. However, most cancers resist to these treatment and relapse after long time treatment. In the recent years, researchers found that there are a subpopulation of cells, playing like stem cells of cancer. As they highly express stemness genes, keep the self-renew ability and are able to differentiate to other non-stemness cancer cells, they are termed as cancer stem cells (CSCs). They stay in quiescent status and resist to traditional chemo-therapy and radio-therapy. Although there is a stochastic model with opinion that all the subpopulation of tumor cells can generate a tumor. However, more and more studies demonstrated recently that the hierarchy model. Only a subpopulation of tumor cells, named cancer stem cell or cancer initiating cells, but the other populations can generate a tumor (Fig. 1) [3].
Researchers isolated CSCs from patients’ tissues and cancer cell lines in different types of cancers, including breast cancer, brain cancer, leukemia, liver cancer, colon cancer and others[4-9]. They are mostly isolated based on the cell surface markers, like CD44+CD24- in breast cancer, CD133+ in brain cancer and CD34+CD38- in Leukemia[8, 10, 9]. Culturing as spheroid bodies in serum-free medium with growth factors and isolating side populations are also used to obtain CSCs[11, 12, 6, 13].
Figure 1. Hierarchy model and Stochastic model
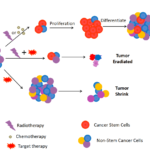
CSCs is the root of cancer. Traditional methods can kill non-stem cancer cells, but not CSCs. The tumors shrink in the short time. However, the remaining CSCs can proliferate, differentiate and generate a relapsed tumor. If the developed drugs can target CSCs, the root of the cancer is eliminated. The tumor lost its ability of self-renew. It will shrink in the long time. If these two types of treatments are combined, both the non-stem cancer cells and CSCs will be eliminated. The tumors will be able to cured (Fig. 2).
Figure 2. Cancer stem cells model
Discussion
Treatment by targeting cancer stem cells
As the traditional cancer therapeutic methods hardly show any effects on cancer stem cells, developing new drugs that targeting to cancer stem cells is vital for cancer therapy. There are many ways to explore the effective drugs targeting to cancer stem cells, such as by screening on active drugs already exists or untested drugs with potential activity, self-renew pathways targeting, destroy the cancer micro-environments and oncolytic viruses treatment (Fig. 3).
High throughput drug screening
High throughput drug screening contributes to find out the specific molecule that most effectively target key signal pathways of cancer stem cells. Then the influence of it to cell morphology and signal pathway of cancer stem cells would be further characterized to determine the effect of the drug. In chronic lymphocytic leukemia, research by high throughput drug screening found that the small molecule Salinomycin could inhibit leukemia through inhibition to Wnt pathway [14]. Besides, researches showed that Salinomycin also exhibited inhibitory effect on other types of cancer stem cells [15]. The small molecules screen on cancer stem cells showed that serotonin and monoamine signal pathways are potential targets of brain cancer therapy [16]. Additional, RNAi pool screening also could be a method to seek effective cancer stem cell targets, thus could cure cancer stem cells by RNA interference or small molecules designed. In brain cancer, TRRAP was explored by RNAi screening which was quantified to related with cell proliferation and self-renew and TRRAP interference could infect tumor formation[17].
Figure 3. Approaches in targeting cancer stem cells
Inhibition of self-renew signal pathway
Signals such as Notch and Hedgehog are intensely related to the self-renewal ability of cancer stem cells. The r-secretase inhibitor of Notch pathway GSIs could prevent cleavage of r-secretase and keeping it to cell membrane thus inhibit Notch signal pathway. Inhibition of Notch signal pathway could inhibit cell proliferation and promote cell differentiation[18-21]. Besides, inhibition of Hedgehog signal pathway could also inhibit tumor effectively, having promised effect in clinic[22]. PTEN/AKT signal pathway also important to cancer stem cells and disable of PTEN activity could activate AKT thus induce activation of Wnt signal pathway which not only have great influence on tumor cell and stem cell, but also on cancer stem cell[14, 23, 24]. Wnt signal pathway is also an important part of cancer stem cells thus high throughput screening of small molecules targeting to Wnt also has great anti-cancer stem cell effect such as Salinomycin on chronic lymphoma leukemia in clinic [14].
Cancer micro-environment
The micro-environment is critical to cancer progression. The immune cells, endothelial cells and stroma cells exhibit different roles in maintaining the cancer. They plays very important role in CSCs maintenance as well. Notch pathway in endothelial cells significant promote the stemness of brain CSCs[18, 20]. Developing the drugs targeting the micro-environment of CSCs will eliminate CSCs, the root of cancer.
Surface marker and ABC transports targeting
Cancer stem cells have high expressed ABC family proteins which help them to pump small molecules out of cells. Besides, they are always in quiescent status thus insensitive to chemical drugs. Additional, cancer stem cells have higher expression of anti-apoptosis proteins such as Bcl-2, XIAP and survivin and stronger DNA repair ability than non-cancer stem cells[25-27]. So cancer stem cells often resistant to chemical drugs and radio-therapy. The cancer stem cell theory proposed recent years considered that cancer therapy killed only non-cancer stem cells could shrink tumor in short period while tumor would relapse after a time due to differentiation and proliferation of cancer stem cells, but on the other hand, if targeting to cancer stem cells and killing them directly, tumor would shrink and disappear due to shortage of self-renewal cancer stem cells even though the therapy hadn’t effect on tumor cells. So it is meaningful to develop drugs targeting to cancer stem cells to promote cancer therapy.
Resistance to anti-apoptosis molecules
High expression of anti-apoptosis molecules is one of the main reason that contributes to resistance of cancer stem cells to traditional therapeutic drugs. The molecules of IAP families such as XIAP and survivin have high expression in cancer stem cells, thus employ them with strong anti-drugs ability[28, 25, 26]. Besides, other anti-apoptosis proteins such as Bcl-2 also play functions on drug resistance.
Oncolytic Virus therapy
Cancer stem cells could pump small molecules out of cells with the help of ABC family proteins on their cell membrane thus show out as insensitive to drugs when treated with small molecule drugs. While virus is a kind of active particle which always connects with receptors of cell membrane through its surface molecules then enters cells in certain way and toxic to tumor cells. Virus has many advantages in cancer stem cell therapy. Firstly, The ABC families of cancer stem cell membranes couldn’t pump virus out[29, 12, 6]. Secondly, some viruses such as adenovirus could infect not only active cells but also the cells in quiescent status such as cancer stem cells[11]. Thirdly, viruses replicate and package themselves in cells and ultimately lyse cells thus cancer stem cells, even though have high level of anti-apoptosis proteins and damage repairing system which render them ability of resistant to small molecule drugs, couldn’t resistant to viruses[30].
Virus is an effective way of treating cancer stem cells. They could be as a vector in non-replicate form and armed with genes or shRNAs targeting to key signal pathway of cancer stem cells or armed with a killing gene to inhibit cancer stem cells[31, 32]. Besides, the oncolytic viruses which only replicate themselves in tumor cells could also armed with killing genes thus have both killing cell and lyse cell ability to enforce the effect of them on cancer stem cells[11].
Oncolytic viruses could specific replicate in tumor cells thus has little side effect. Besides, they could infect and lyse cancer stem cells directly due to characteristics of themselves. So oncolytic virus is a good way targeting to cancer stem cells. In present, many oncolytic viruses have been used for cancer therapy including DNA viruses such as adenovirus, poxviruses, herpes viruses and RNA viruses such as retroviruses[33-38]. Each virus has its own advantages and disadvantages and has important role in cancer therapy, besides they could kill cancer stem cells as well. The targeting therapeutic effect of different viruses on cancer stem cells would be detailed as followers.
Conclusion
Cancer stem cells were the main obstacles of traditional cancer therapeutic methods. High throughput screening could help to explore drugs specific targeting to cancer stem cells, besides, molecules designed targeting to key signal pathways of cancer stem cells could also help to overcome the obstacles of resistance to drugs. Virus therapy has many advantages compared with small molecule drugs, such as viruses couldn’t be pumped out by cancer stem cells, don’t easy induce resistance to them and have effect on even cells in quiescent status, while combined viruses with gene such as oncolytic adenoviruses armed with genes targeting to self-renewal related pathways of cancer stem cells had better effect on cancer therapy. So to eliminate the origin of tumor cells radically by combination of many methods targeting to cancer stem cells could bring great effect on cancer therapy.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
None.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65(2):87-108. doi:10.3322/caac.21262.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5-29. doi:10.3322/caac.21254.
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133-43. doi:10.1038/nrc3184.
- Yin S, Li J, Hu C, Chen X, Yao M, Yan M et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120(7):1444-50. doi:10.1002/ijc.22476.
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111-5. doi:nature05384 [pii]10.1038/nature05384.
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267-84. doi:10.1146/annurev.med.58.062105.204854.
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396-401. doi:nature03128 [pii]10.1038/nature03128.
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821-8.
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645-8. doi:10.1038/367645a0.
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983-8. doi:10.1073/pnas.05302911000530291100 [pii].
- Yang Y, Xu H, Huang W, Ding M, Xiao J, Yang D et al. Targeting lung cancer stem-like cells with TRAIL gene armed oncolytic adenovirus. J Cell Mol Med. 2015;19(5):915-23. doi:10.1111/jcmm.12397.
- Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827-33. doi:67/10/4827 [pii]10.1158/0008-5472.CAN-06-3557.
- Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3(10):801-6. doi:nmeth926 [pii]10.1038/nmeth926.
- Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 2011;108(32):13253-7. doi:1110431108 [pii]10.1073/pnas.1110431108.
- Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett. 2011;311(1):113-21. doi:S0304-3835(11)00430-7 [pii]10.1016/j.canlet.2011.07.016.
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4(6):568-80. doi:10.1016/j.stem.2009.03.014.
- Wurdak H, Zhu S, Romero A, Lorger M, Watson J, Chiang CY et al. An RNAi screen identifies TRRAP as a regulator of brain tumor-initiating cell differentiation. Cell Stem Cell. 2010;6(1):37-47. doi:10.1016/j.stem.2009.11.002.
- Deng SM, Yan XC, Liang L, Wang L, Liu Y, Duan JL et al. The Notch ligand delta-like 3 promotes tumor growth and inhibits Notch signaling in lung cancer cells in mice. Biochem Biophys Res Commun. 2017;483(1):488-94. doi:10.1016/j.bbrc.2016.12.117.
- Li D, Masiero M, Banham AH, Harris AL. The notch ligand JAGGED1 as a target for anti-tumor therapy. Frontiers in oncology. 2014;4:254. doi:10.3389/fonc.2014.00254.
- Guo H, Lu Y, Wang J, Liu X, Keller ET, Liu Q et al. Targeting the Notch signaling pathway in cancer therapeutics. Thoracic cancer. 2014;5(6):473-86. doi:10.1111/1759-7714.12143.
- Xu X, Liu RF, Zhang X, Huang LY, Chen F, Fei QL et al. DLK1 as a potential target against cancer stem/progenitor cells of hepatocellular carcinoma. Mol Cancer Ther. 2012;11(3):629-38. doi:10.1158/1535-7163.MCT-11-0531.
- Merchant AA, Matsui W. Targeting Hedgehog–a cancer stem cell pathway. Clin Cancer Res. 2010;16(12):3130-40. doi:1078-0432.CCR-09-2846 [pii]10.1158/1078-0432.CCR-09-2846.
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS biology. 2009;7(6):e1000121. doi:10.1371/journal.pbio.1000121.
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226-35. doi:10.1016/j.stem.2009.01.007.
- Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer cell international. 2016;16:49. doi:10.1186/s12935-016-0326-1.
- Cheng XJ, Lin JC, Ding YF, Zhu L, Ye J, Tu SP. Survivin inhibitor YM155 suppresses gastric cancer xenograft growth in mice without affecting normal tissues. Oncotarget. 2016;7(6):7096-109. doi:10.18632/oncotarget.6898.
- Ahangar P, Sam MR, Nejati V, Habibian R. Treatment of undifferentiated colorectal cancer cells with fish-oil derived docosahexaenoic acid triggers caspase-3 activation and apoptosis. Journal of cancer research and therapeutics. 2016;12(2):798-804. doi:10.4103/0973-1482.157326.
- Jeong JY, Kang H, Kim TH, Kim G, Heo JH, Kwon AY et al. MicroRNA-136 inhibits cancer stem cell activity and enhances the anti-tumor effect of paclitaxel against chemoresistant ovarian cancer cells by targeting Notch3. Cancer Lett. 2017;386:168-78. doi:10.1016/j.canlet.2016.11.017.
- Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V et al. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148(4):1797-803. doi:en.2006-1553 [pii]10.1210/en.2006-1553.
- Ribacka C, Hemminki A. Virotherapy as an approach against cancer stem cells. Curr Gene Ther. 2008;8(2):88-96.
- Kanai R, Rabkin SD, Yip S, Sgubin D, Zaupa CM, Hirose Y et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104(1):42-55. doi:10.1093/jnci/djr509.
- Jang JY, Kim MK, Jeon YK, Joung YK, Park KD, Kim CW. Adenovirus adenine nucleotide translocator-2 shRNA effectively induces apoptosis and enhances chemosensitivity by the down-regulation of ABCG2 in breast cancer stem-like cells. Experimental & molecular medicine. 2012;44(4):251-9. doi:10.3858/emm.2012.44.4.019.
- Yang Y, Xu H, Shen J, Yang Y, Wu S, Xiao J et al. RGD-modifided oncolytic adenovirus exhibited potent cytotoxic effect on CAR-negative bladder cancer-initiating cells. Cell death & disease. 2015;6:e1760. doi:10.1038/cddis.2015.128.
- Thirukkumaran C, Morris DG. Oncolytic viral therapy using reovirus. Methods Mol Biol. 2009;542:607-34. doi:10.1007/978-1-59745-561-9_31.
- Short JJ, Curiel DT. Oncolytic adenoviruses targeted to cancer stem cells. Mol Cancer Ther. 2009;8(8):2096-102. doi:1535-7163.MCT-09-0367 [pii]10.1158/1535-7163.MCT-09-0367.
- Eriksson M, Guse K, Bauerschmitz G, Virkkunen P, Tarkkanen M, Tanner M et al. Oncolytic adenoviruses kill breast cancer initiating CD44+CD24-/low cells. Mol Ther. 2007;15(12):2088-93. doi:10.1038/sj.mt.6300300.
- Norman KL, Coffey MC, Hirasawa K, Demetrick DJ, Nishikawa SG, DiFrancesco LM et al. Reovirus oncolysis of human breast cancer. Human gene therapy. 2002;13(5):641-52. doi:10.1089/10430340252837233.
- Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98(11):6396-401. doi:10.1073/pnas.101136398.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/