J Med Discov (2017); 2(1):jmd17010; doi:10.24262/jmd.2.1.17010; Published April 13th, 2017.
An Optimized method for construction of Epstein-Barr virus-transformed immortalized lymphoblastoid cell lines
Chang Liu1,#, Hao Liu1,#, Li Zhang1,#, Ni Deng2
1Pharmacy of School, University of Missouri-Kansas City, MO 64108, United States
2The First people’s hospital of Changzhou, Changzhou 213000, China
* Correspondence: Chang Liu, School of Pharmacy, University of Missouri-Kansas City, 2464 Charlotte Street, Kansas City, MO 64108, United States. Email: cl7p9@mail.umkc.edu.
# Chang Liu, Hao Liu and Li Zhang contributed equally to this work.
Introduction
Advancement in biotechnology research has achieved partly by the availability of cell lines from biological material, providing supply of cells with ideal genotypes and phenotypes [1, 2]. Establishment of lymphoblastoid cell lines (LCLs) is one of such major achievements, which has a variety of applications such as presenting antigens in the immunologic assays, generation of human monoclonal antibodies and providing an unlimited source with respect to limitation of primary biologic materials [3]. Infection of B lymphocytes with Epstein-Barr virus (EBV) is a classic method to construct immortalized LCLs in vitro [4].
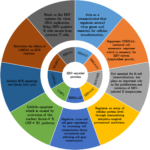
EBV, a human gamma herpesvirus, infects preferentially human B lymphocytes. However, EBV also infects other cells such as epithelial cells and T cells in some circumstances [4]. There are over ~90% of human kind infected with EBV in the world. In the peripheral blood, the virus persists in the resting memory B lymphocytes, expressing no viral genetic information, which is called latency program. They are invisible to the host’s immune system. However, this behavior is at odds with infected B lymphocytes in vitro. In vitro EBV infection has no preference but results in B lymphocytes activation via the concerted expression of latent genes under the modulation of the transcription factor Epstein-Barr nuclear antigen 2 (EBNA2) [5]. Latently infected B lymphocytes has over nine EBV-encoded proteins. As shown in Fig. 1, The EBNAs include EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C and EBNA-LP and the latent membrane proteins include LMP1, LMP2A and LMP2B [6]. Among those EBNAs, EBNA 2 is the first virus protein, expressed after infection of B lymphocytes in vitro. EBNA 2 is essential for cellular transformation. It is a trans-activator, regulating several virus genes including LMP1 and LMP2A, playing important roles in progress of full activation of B lymphocytes. LMP1 mimics the constitutive activation of CD40, which has a critical role in the activation and differentiation of B lymphocytes, whereas LMP2A induces the activation of a B-cell receptor (BCR) and provides a survival signal for B lymphocytes [4, 7]. EBV infection results in activation and proliferation of B lymphocytes and transformation of B cells into LCL, providing a continuous source for further applications. Importantly, EBV-mediated transformation results in fewer genetic changes because the virus remains in the episomal form inside the B lymphocytes. Only a few viral genes are apparently expressed inducing minimal change in genome. However, in vitro study, only small proportion of infected B cells, around 1~3 %, can be transformed to LCL [8].
Fig.1 Functions of the EBV-encoded proteins
A variety of methods have been reported to boost the efficiency of EBV-mediated immortalization. Early methods have included the use of mitogens such as lipopolysaccharide (LPS), phytohemagglutinin (PHA), and pokeweed mitogen (PWM) [9-11]. LPS results in a 300-500% increase of the number of transformation events. Pretreatment of cord blood leukocytes with PHA increases the number of transformation events observed by 0.5-fold. Another method is that gamma-irradiated fibroblast type cells have been used as feeder layers to increase the efficiency of EBV-mediated immortalization [12]. In addition, cyclosporin A (CSA) has been used to diminish B-cell regression in proliferation by inhibiting cytotoxic T-cell action [13]. Furthermore, by modifying the standard method for preparation of high titer EBV stock, and the infection frequency of EBV, the efficiency of EBV-mediated immortalization could be improved [14].
Most of these methods are available currently in EBV-mediated immortalization. However, the length of time from initiation of culture to immortalization is the biggest obstacle to be solved. In recent, Lu and Sun optimized the EBV transformation by multiple aspects including preparation of titer EBV stock, adding cytokines, co-culture with the feeder cells, and the change of cell density [15]. The encouraging results demonstrate that the efficiency of the optimized methods was approximately 7.8% (0.6%–20%). This result is three-fold higher than efficiency of the improved EBV transformation method (2.4% (0.4%–11%)), which is dramatically higher than conventional EBV transformation methods (10-6-10-4). This study also shows some interesting results. First, transformation efficiency positively correlated with the EBV titer, but not in a linear relationship. Second, optimal spin-fecion conditions significantly increases EBV transformation efficiency. Long-term immortalization of B cells, efficiency of immortalization with centrifugation at 500 g produced about 3-fold than that of with centrifugation at 250 g. Third, among cytokines including CD40L, IL-2, IL-4, IL-21, INF-γ, BAFF, CpG, and anti-IgM F(ab)2, BAFF is the most important cytokine for long-term proliferation of B lymphocytes. Proliferation of B lymphocytes stimulated with BAFF or IL-2 is slightly faster, whereas proliferation of B lymphocytes reduces by stimulating with other activators and cytokines.
It is known that CD40L co-stimulation displays a promoting effect on EBV transformation of short-term culture of B cells in vitro. However, it is still unclear that the CD40L co-stimulation system has an improving effect EBV-mediated transformation for long-term B cell culture. In this study, Lu and Sun demonstrate that 3.5×, 40.2×, 84.9× and 211.3× fold increases of immobilized B cells were recorded on days 4, 8, 12 and 16 in the CD40L co-stimulation system, whereas there are only 1.3×, 4.1×, 13.4× and 27× increases in B cells were recorded on days 4, 8, 12 and 16 without CD40L co-stimulation. Basing on that, Lu and Sun further investigate that effects of densities of initial PBMCs and feeder cells on the efficiency of CD40 co-stimulation, indicating that the optimal cell densities for initial PBMCs and feeder cells are 4×105/ml and 4×104/well (six-well plate), respectively.
Furthermore, basing on optimized EBV immortalization method, an immortalized human naïve B cell library with the diversity of BCR repertoire at about 6×106 was generated. This naïve B cell library is useful for isolation of viral specific naïve B cell lines for further applications.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
None
References
- Song, L.Q., et al., Nanotopography promoted neuronal differentiation of human induced pluripotent stern cells. Colloids and Surfaces B-Biointerfaces, 2016. 148: p. 49-58.
- Shi, L., K. Wang, and Y. Yang, Adhesion-based tumor cell capture using nanotopography. Colloids and Surfaces B-Biointerfaces, 2016. 147: p. 291-299.
- Hussain, T. and R. Mulherkar, Lymphoblastoid Cell lines: a Continuous in Vitro Source of Cells to Study Carcinogen Sensitivity and DNA Repair. Int J Mol Cell Med, 2012. 1(2): p. 75-87.
- Kuppers, R., B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol, 2003. 3(10): p. 801-12.
- Klein, G., E. Klein, and E. Kashuba, Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun, 2010. 396(1): p. 67-73.
- Babcock, G.J., D. Hochberg, and A.D. Thorley-Lawson, The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity, 2000. 13(4): p. 497-506.
- Ma, S.D., et al., Latent Membrane Protein 1 (LMP1) and LMP2A Collaborate To Promote Epstein-Barr Virus-Induced B Cell Lymphomas in a Cord Blood-Humanized Mouse Model but Are Not Essential. J Virol, 2017. 91(7).
- Younesi, V., et al., Assessment of the effect of TLR7/8, TLR9 agonists and CD40 ligand on the transformation efficiency of Epstein-Barr virus in human B lymphocytes by limiting dilution assay. Cytotechnology, 2014. 66(1): p. 95-105.
- Henderson, E., et al., Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology, 1977. 76(1): p. 152-63.
- Bird, A.G., et al., Characteristics of Epstein-Barr virus activation of human B lymphocytes. J Exp Med, 1981. 154(3): p. 832-9.
- Sun, W., et al., Ilexgenin A, a novel pentacyclic triterpenoid extracted from Aquifoliaceae shows reduction of LPS-induced peritonitis in mice. Eur J Pharmacol, 2017.
- Manor, E., Human plasma accelerates immortalization of B lymphocytes by Epstein-Barr virus. Cell Prolif, 2008. 41(2): p. 292-8.
- Pelloquin, F., J.P. Lamelin, and G.M. Lenoir, Human B lymphocytes immortalization by Epstein-Barr virus in the presence of cyclosporin A. In Vitro Cell Dev Biol, 1986. 22(12): p. 689-94.
- Oh, H.M., et al., An efficient method for the rapid establishment of Epstein-Barr virus immortalization of human B lymphocytes. Cell Prolif, 2003. 36(4): p. 191-7.
- Shiqiang Lu, Zehua Sun, and Mei-yun Zhang, Generation of immortalized human naïve B cell libraries by optimized EBV transformation. J Med Discov, 2017. 2(1): jmd17007.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/