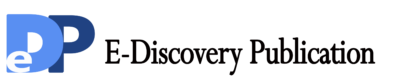J Med Discov (2017); 2(1):jmd17007; doi:10.24262/jmd.2.1.17007; Received March 31st, 2017, Accepted April 9th, 2017, Published April 12th, 2017.
Generation of immortalized human naïve B cell libraries by optimized EBV transformation
Shiqiang Lu1,#, Zehua Sun2,#,*, Mei-yun Zhang3,4,*
1GenScript USA Inc., 860 Centennial Ave, Piscataway, NJ, 08854, USA
2Department of Medicine, National Jewish Health, 1400 Jackson Street, Denver, CO, 80206
3Department of Microbiology, the University of Hong Kong, Hong Kong, 999077
4Liver Disease Institute, Shenzhen Third People’s Hospital, Shenzhen 518112, China
# Shiqiang Lu and Zehua Sun contributed equally to this work.
*Corresponding to: Zehua Sun, PhD, Department of Medicine, National Jewish Health. Denver, CO, United States, 80206. Email: sunz@njhealth.org. OR Mei-Yun Zhang, PhD, Department of Microbiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong. 21 Sassoon Road, Pokfulam, Hong Kong, China. Email: zhangmy@hku.hk.
Abstract
Epstein–Barr virus (EBV) transformation is a useful tool in generating immortalized B cells. However, the B cell immortalization efficiency is quite low in conventional method of EBV transformation (1%) and improved EBV transformation (2.4%). Here we optimized the EBV transformation by multiple aspects including viral concentration, cytokines, co-culture with feeder cells, cell density. The results showed that the efficiency of the methods was approximately 7.8% (0.6%–20%), which was three folds higher than that of the improved EBV transformation method of 2.4% (0.4%–11%). Based on the optimized method, an immortalized human naïve B cell library with the diversity of BCR repertoire at about 6×106 was generated from health donors. This naïve B cell library is useful for isolation of viral specific naïve B cell lines for the maturation study in vitro. Our results provide knowledge in optimized EBV transformation and naïve B cell library construction.
Keywords: Technology, EBV transformation, B cell immortalization, human naïve B cell library
Introduction
To generate B cell libraries for isolating antigen-specific B cells, two requirements have to be fulfilled. One is the immortalization of human B cells for long-term culture; the other is that high immortalization efficiency is preferred to maintain the diversity of human B cells to recapitulate the B cell population in vivo. Immortalization of B cells by EBV transformation is the only feasible solution for long-term culture of human B cells [1-3]. EBV has successfully caused latent infection in over 95% of individuals worldwide, which preferentially infected human B cells and led to multiple distinct latency patterns. EBV-induced transformation can immortalize human B cells into lymphoblastoid cells. Meanwhile, the EBV genome does not integrate into the genome of host cells. Instead, it exists as extrachromosomal copies (viral episomes). lymphoblastoid cells are usually assumed as an activated state of B cells with high expression of CD23, CD80, CD86, CD70 and adhesion molecules, such as LFA1, LFA3, and ICAM1, on the cell surface [2]. By contrast, these molecules are not expressed or only marginally expressed on resting B cells. However, upon activation by antigens or mitogens, the expression of these activated markers is upregulated. EBV transformed human B cells resemble the physiological state of B cells in germinal center. The activation state is associated with the immortalization of human B cells in vitro.
Generally, three events are necessary for full activation of B cells [4]. The first event is the single transduction from the B cell receptors after binding with antigens. The second event is the engagement of CD40 with its ligand (CD40L) expressed on T cells. The third event is the involvement of other receptors on B cells, including different Toll-like receptors (TLRs). EBV encodes two additional membrane proteins, latent membrane protein 1 (LMP1) and latent membrane protein 2 (LMP2A). LMP1 mimics the constitutive activation of CD40 [5], whereas LMP2A induces the activation of BCR [6]. The involvement of unknown viral components or other coinfection pathogens serve as the third event in B cells activation [4]. Although the exact mechanism responsible for B cell immortalization remains unclear, current studies showed that it is a complex and dynamic process containing multiple stages. Human B cells are readily transformed by EBV. Numerous human antibodies have been identified by this method. However the efficiency of the conventional EBV transformation method is quite low (10-6-10-4) [7]. Little progress has been made in the 20 years following the discovery of EBV. Until the discovery of TLR-9 agonist CpG, which shows great synergetic effect in EBV-induced transformation, this method has been successfully used to isolate antibodies against SARS [8], HIV-1 [9] and HCMV [10]. EBV transformed B cells can fuse with partner cells to form stable hybridoma cell lines, which facilitates the production of human antibodies [11].
Other polyclonal activators have also been attempted to further increase EBV-induced transformation of human B cells besides CpG. R848, a strong agonist for both TLR7 and TLR8, can efficiently activate B cells but show little synergetic effect on EBV transformation. Moreover, LPS, the agonist of TLR4, has been long recognized as a strong B cell activator. However, its effects on EBV induced transformation are quite conflicting. Additionally, certain cytokines can also increase EBV transformation efficiency, such as B cell-activating factor (BAFF), IL-2, IL-10, IL-21, APRIL, INF-γ, IL-4 and IL-15. Another breakthrough came from B cell short-term culture in vitro by CD40L-expressing feeder cells. In the early 1990s, an in vitro system for short-term activation of human B cells was established using CD40L-expressing fibroblast cells
(L-CD40L) as feeder cells. This method was successfully used to generate autologous CD40-activated B cells as an alternative source of highly efficient antigen-presenting cells for adoptive immunotherapy [12]. A new culture system was subsequently generated based on the new feeder cells, which express both CD40L and BAFF on the cell surface [13]. This system can greatly increase the short-term proliferation of mouse B cells compared with the early system. However, whether L-CD40L enhances EBV transformation remains unknown. Irradiated autologous T cells were recently used for short-term culture of B cells, which allowed direct neutralization screening of supernatants that led to the discovery of some very potent and bread bNAbs against HIV-1, such as PG9, PG16 and PGT151–158, etc [14-17]. Moreover, autologous T and CpG can work synergistically to increase EBV transformation, leading to the isolation of neutralizing antibodies against influenza virus and also autoreactive antibodies [8, 18]. Even so, the efficiency of EBV transformation is still quit low in the presence of autologous T cells. To further increase the efficiency of EBV transformation for the construction of an immortalized human naïve B cell library, efforts have been made to improve the EBV transformation efficiency in this study. Based on this optimized EBV transformation method, an immortalized human naïve B cell library with the diversity of BCR repertoire at about 6×106 was generated from the naïve B cells isolated from 16 health donors.
Materials and Methods
Cell lines
The cell lines used in this study and their organism and applications are included in Table 1.
Table 1. Cell lines and their origin
| Cell lines | Organism and Tissue | applications |
| B95-8 | Marmoset lymphocyte | producing EBV stock |
| 293T | human embryonic kidney | Expression of fusion cytokines in small quantity |
| Hela | Human cervix epithelial cells | Generation of CD40L expressing feeder cells; host cells for titration of lentivirus |
Growth medium for cell culture
Dulbecco’s Modified Eagle Medium (DMEM) growth medium for 293T, Hela, NIH3T3 and TZM-bl: DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/ml penicillin, and 100 µg/ml streptomycin. RPMI 1640 growth medium for B95-8, DG-75 and Raji: RPMI 1640 medium supplemented with 10% FBS, 100 units/ml penicillin, 100µg/ml streptomycin and 50μM β-mercaptoethanol. Growth medium for EBV transformation: Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% FBS, 0.66 µg/ml cyclosporin A (CsA), 100 units/ml penicillin, 100 µg/ml streptomycin and 50μM β-mercaptoethanol. Growth medium for CD40L-B cells: IMDM supplemented with 10% FBS, 0.66 µg/ml CsA, 2 µg/ml IL-4, 5 µg/ml insulin, 30 µg/ml transferrin, 100 units/ml penicillin, 100 µg/ml streptomycin and 50μM β-mercaptoethanol.
Virus and plasmids
EBV-producing marmoset B cell line (B95-8), commonly used as a source of EBV for infection and immortalization of human B cells, was kindly provided by Prof. G.S.W. Tsao of the Department of Anatomy, Li Ka Shing Faculty of Medicine, HKU. Mammalian expression vector (pcDNA 3.1(-)) was from Life Technologies (Catalog number: V795-20) and used for the cloning and expression of human CD40L on mammalian cells.
Preparation of concentrated virus stock
To prepare infectious EBV stocks as previously reported with minor modifications [19], EBV-producing marmoset cells (B95.8) were seeded at 106 cells/ml and incubated at 37 °C and 5% CO2 for 4 days in IMDM complemented with 10% heat-inactivated FBS, 1% penicillin-streptomycin, and 35 ng/ml 12-O-tetradecanoylphorbol-13-acetate (TPA)/ml. After centrifugation at 800g for 5min, the supernatant was passed through a 0.45µm filter. To concentrate the virus, EBV-containing supernatant was loaded in Centricon Plus-70 units (100,000 MW cut-off, Millipore) and centrifuged at 3200 g for 45 min with the minimal retentate volume left. The virus-containing concentrate was resuspended with fresh IMDM medium up to 1/10 of the original volume. Aliquots of 1ml per vial were preserved at -80 °C .
Titration of EBV by transformation units
Freshly purified primary B cells (105) in 500 μl of RPMI1640 growth medium were cultured in a 24-well plate. Different amounts of EBV supernatant at 1, 5, 25, 125, and 625 μl were added in each well. Finally, fresh growth medium was added to each well to obtain a total volume of up to 1.2ml. Cells were incubated at 37 °C and 5% CO2 for three days and then analyzed by flow cytometry with forward and side scatter. A lymphoblastoid cell was assumed to represent 1 unit of transforming EBV.
Isolation of human naïve B cells
PBMCs were isolated from peripheral blood buffy coats from healthy donors by Ficoll density centrifugation. B cells were further purified with Human B Cell Isolation Kit (Miltenyi Biotech, Germany). Non-B cells were indirectly deleted with magnetical microbeads after they were labeled with a cocktail of biotin-conjugated monoclonal antibodies. Memory and naïve B cells were separated by CD27 microbeads (Miltenyi Biotech, Germany). Moreover, the purity of the cell populations was verified by flow cytometry.
New B cell immortalization method by EBV infection
Purified naïve B cells were suspended in 3 ml of IMDM supplemented with 10% heat-inactivated fetal bovine medium, 100 units/ml penicillin, 100 µg/ml streptomycin, 0.66 µg/ml CsA, 2 µg/ml CpG2006, 25 ng/ml BAFF, 2 µg/ml LPS and 5 ng/ml IL-2. About 3ml of concentrated EBV-containing supernatant was added to the cells suspension and mixed well. The suspension was centrifuged at 500 g for 60 min at room temperature and then suspended at 106 /ml. Cells were seeded into 96-well U-bottom plates at 200 µl/well. Plates were incubated at 37 °C and 5%CO2, and half-medium was changed every 7 days. After three weeks of culture cells were transferred to T75 flask for further culture.
B cell culture in CD40L system
CD40L feeder cells were irradiated at 80 Gy and seeded into six-well plates at 0.2×104 cells/well in DMEM containing 10% FBS, 100 units/ml penicillin and 100 µg/ml streptomycin. After overnight culture (>6 h), plates were washed with PBS for three times before use as feeder cells. Meanwhile, PBMCs were freshly isolated from peripheral blood buffy coats by Ficoll density centrifugation and then resuspended at 5×105-106/ml in CD40L-B growth medium containing IMDM supplemented with 10% FBS, 0.66 µg/ml CsA, 50 μM β-mercaptoethanol, 2 µg/ml IL-4, 5 µg/ml insulin, 30 µg/ml transferrin, 100 units/ml penicillin and 100 µg/ml streptomycin. Finally PBMCs were seeded in six-well plates in the presence of irradiated CD40L feeder cells and incubated at 37 °C and 5% CO2. Feeder cells were replaced with fresh irradiated ones every 4 days. After day 7, the concentration of cells should be reduced to 5×105 for further subculture. Generally CD19+B cells accounted for over 70% in total PBMC by then.
Proliferation assay
CFSE is a commonly used assay to evaluate cell proliferation of lymphocytes in vitro and in vivo by labeling with dye dilution to track cell multiple generations by flow cytometry. The optimal concentration of CFSE for B cell staining was 5 μM as determined by tracking five cell generations. The CFSE manual for labeling cells in suspension was strictly followed. In brief, B cells were incubated with 5 μM CFSE at room temperature for 20 min without light. Cells were washed five times in the original staining volume of growth medium before treatmentwith different stimulators. Finally, cells proliferation was examined by flow cytometry for FITC signal on day 6.
Evaluation of EBV-induced immortalization efficiency by lymphoblastoid cell outgrowth
After EBV infection by either conventional EBV transformation method or EBV spinfection, B cells were resuspended in EBV transformation medium (IMDM supplemented with 10% FBS, 0.66 µg/ml CsA, 50 μM β-mercaptoethanol, 100 units/ml penicillin, and 100 µg/ml streptomycin). Cells were accounted and seeded into U-bottom 96-well plates at five fold serial dilutions. For example, the wells in the first row contained 8,000 cells, and the next row contained 1,600 cells, and so forth. The 96-well plates were incubated at 37 °C and 5% CO2 with half-medium changed every 6 days. On day 18, the cells were observed under a light microscope as lymphoblastoid cell outgrowth could be clearly observed by then. One lymphoblastoid cell cluster was assumed to be derived from the outgrowth of one B cell, which was regarded as one immortalization event. The immortalization efficiency could be readily estimated from the rows with the lowest number of lymphoblastoid cell outgrowth.
Results
TPA-induced release, high concentration of EBV-containing supernatant and spinfection greatly enhanced EBV transformation efficiency
The most interesting characteristic of EBV infection is its ability to immortalize human B cells in vitro. WT EBV derived from B95.8 cells was used in this study. Human B cells were proofed readily transformed by EBV infection via conventional protocol, which led to the steady proliferation of lymphoblastoid cells that could be readily detected by flow cytometry scatter analysis. EBV titration is usually determined by two methods. One is to detect the EBV genome by quantitative real-time PCR, which is a routine method for most recent published papers because of its good repeatability and accuracy. However, the number of EBV gene copies does not simply equal viral viability. The other way is by viral transforming units, which evaluate EBV transformation ability by infection of primary B cells. In brief, freshly isolated B cells were incubated in the presence of serially diluted EBV-containing supernatants. The emergence of lymphoblastoid cells was analyzed by flow cytometry on day 3. A logarithmically linear relation was readily detected between EBV doses and lymphoblastoid cells numbers (Figure 1). Accordingly, the titer of EBV stock could be calculated by the formula:
EBV transforming units/ml = 2×105 × (% of LCL cells/100)/volume of supernatant in ml.
Figure 1 Titration of EBV stock by transforming units. (A) Emergence of lymphoblastoid cells was measured by flow cytometry. (B) The number of lymphoblastoid cells was logarithmically linear dependent on the EBV dose.
Figure 2 TPA-induced release of EBV virus followed by 10× concentration EBV-containing supernatant greatly enhanced EBV transformation efficiency. (A) Evaluation of B cell immortalization efficiency by flow cytometry. (B) Increased EBV transformation efficiency by TPA
In our experience, this method was readily repeatable. However, variations may exist when different human B cells are used. Consistent with previous records, EBV stocks were usually in low titers, which limited the transformation efficiency of human B cells. To solve this problem, we optimized the preparation of EBV stocks with high titration. First, EBV-containing supernatants were harvested with conventional manual as control. Meanwhile, B95.8 cells were stimulated with TPA or anti-huIgM F(ab)2 for 4 days, followed by the conventional procedure for the preparation of EBV stocks. The titration of EBV stock was evaluated with the method described as above. EBV titers were nearly five times higher when treated with TPA (Figure 2). In addition, TPA showed a slightly better stimulation for releasing of EBV than anti-hu IgM F(ab)2. Given economical consideration, TPA was used to prepare EBV stocks in the later experiments. EBV titer could be further increased by the concentration of EBV-containing supernatants via high-speed centrifugation as described previously [19]. Besides, two additional benefits existed for the viral concentration. First, the remaining TPA was removed from the supernatant because it could affect the transformation of B cells during EBV infection. Second, viral stocks were replaced with fresh growth medium, which may result in better survival of primary human B cells in the early infection stage of human B cells. As expected, the results showed about two folds increase in EBV transformation efficiency after the viral concentration.
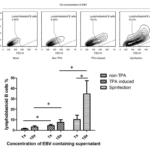
Figure 3 Effect of relative centrifugal force, time and temperature on short-term and long-term EBV transformation efficiency. (A) Effect of centrifugal force on EBV transformation efficiency on day 3. (B) Effect of centrifugal time on EBV transformation efficiency on day 3. (C) Effect of centrifugal force on EBV transformation efficiency on day 15. (D) Effect of centrifugal time on EBV transformation efficiency on day 15. (E) B cell growth curve with or without spinfection infected with different EBV doses. (F) Effect of centrifugal temperature on EBV transformation efficiency.
Although TPA stimulation followed by EBV supernatant concentration could significantly increase EBV titer, B cell transformation efficiency was still very low. Previous literature showed that low-speed concentration of cells with HIV-1 virus greatly enhances viral infection efficiency. This method (termed spinfecion) has been well used to increase the infection efficiency of other envelope viruses. However, the protocols of spinfection differ among various studies for EBV transformation. Besides, only transient infection efficiency instead of long-term growth of B cells was examined in those studies. To generate B cells libraries for cells panning, long-term culture of lymphoblastoid cells is required. Thus, we examined the optional conditions responsible for long-term culture of B cells after EBV transformation. For this purpose, B cells were infected with EBV at MOI 0.5 in 24-well plates. The plates were centrifuged with different centrifugal forces, times and temperatures. The transformation efficiency was assessed on days 3 and 15. For short-term EBV transformation efficiency measured on day 3, centrifugations at 250, 500, 1000 and 1250 g all contributed equally to the increase in EBV transformation efficiency (Figure 3A). Meanwhile, centrifugations at different time spans also showed a similar increase in EBV transformation efficiency (Figure 3). Interestingly, significant differences appeared when the growth of B cells was examined on day 15. The results showed that B cells proliferated best when centrifuged at 500 g. By contrast, B cells proliferated poorly when centrifuged at 1250 g (Figure 3C). Moreover, B cell proliferated best when centrifuged between 30 and 60min (Figure 3D). We speculated that centrifugation at higher force (>500g) and longer time (>60min) possibly impaired the viability of primary B cells, which was responsible for the reduction in B cell proliferation. In addition, spinfection at different temperatures did not show any difference for both short-term and long-term EBV transformation efficiency (Figure 3F). Enhanced B cells outgrowth with or without centrifugation was confirmed by infection of B cells with different EBV doses (Figure 3E). All these results showed that spinfection could increase both short-term EBV transformation efficiency and long-term B cell proliferation when centrifuged at 500 g for 60 min.
Effect of cytokines on EBV infection
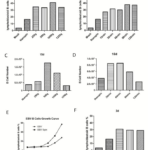
Since the discovery that EBV can transform human B cells in vitro, numerous cytokines and activators have been investigated to obtain higher transformation efficiency. Such efforts included soluble CD40L, IL-2, IL-4, IL-21, INF-γ, BAFF, CpG, and anti-IgM F(ab)2. However, only short-term EBV transformation efficiency was examined in previous studies by 3H-thymidine or CFSE assay between days 3 and 6. Thus, we aimed to determine whether EBV transformation efficiency can be improved in the presence of different B cells activators for long-term culture in vitro. Consistent with previous finding, CpG worked best for B cell stimulation tested on day 7 with or without EBV infection. B cells stimulated with BAFF or IL-2 proliferated slightly faster, whereas B cells showed reduced proliferation when treated with other activators and cytokines (Figure 4A). However, B cells stimulated with BAFF and LPS demenstrated good proliferation on day 21 (Figure 4C). The growth curve of B cells treated with different activators in the presence or absence of EBV infection is showed in Figure 4D and 4E. We then examined whether early stimulation of B cells contributes to enhanced B cell proliferation by adding BAFF or LPS only in the first 2 days after EBV infection. The results showed that early stimulation of B cells with BAFF was sufficient to enhance EBV transformation efficiency. Therefore, early presence of BAFF could aid in better outgrowth of EBV-transformed B cells for long-term in vitro culture, whereas other activators could improve EBV transformation efficiency only in short-term culture system(<7 days).
Figure 4 BAFF enhanced long-term EBV-induced B cell proliferation. (A) B cell growth on day 3. (B) B cell growth on day 14. (C) B cell growth on day 21. (D) B cell growth curve with EBV infection. (E) B cell growth curve without EBV infection. (F) Early presence of BAFF enhanced long-term B cell proliferation.
Evaluation of B cell immortalization efficiency by lymphoblastoid cell outgrowth
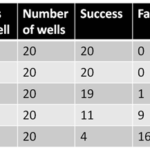
Given that the conditions of EBV transformation method have been optimized for better B cell proliferation in a long-term culture, we then examined B cell immortalization efficiency using lymphoblastoid cell outgrowth assay (Table 2). In brief, B cells were counted and dispensed into 96-well U-bottom plates in a five-fold dilution series about 12 h after EBV infection. Cells were incubated at 37 °C and 5% CO2 for 21 days. By then, lymphoblastoid cell outgrowth was readily visible under light microscopy. EBV immortalization efficiency was estimated based on the assumption that one lymphoblastoid cell cluster resulted from the immortalization of at least one B cell. Thus, the efficiency was calculated from rows containing the lowest number of cells per well in which lymphoblastoid cell proliferation was consistently observed. It was expressed as one immortalization event per number of cells originally dispensed into the well. The results showed that the efficiencies of the improved EBV immortalization methods were 2.4% (0.4%-11%), which was much higher than that of convention EBV transformation methods (10-6-10-4). The diversity of the B cell library was approximately 2.4×106 starting from 108 B cells. The efficiency was still quite low, which could lead to the loss of most B cells during the generation of the human B cell library in the first few weeks after EBV transformation. However, the generation of immortalized human B cells may be possible based on the improved EBV transformation procedure.
Table 2. Cell density and success rate for the establishment of LCLs by EBV transformation.
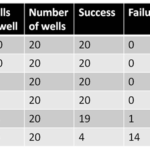
Table 3. Cell density and success rate for the establishment of LCLs by combining EBV transformation and CD40L stimulation
CD40L system for long-term B cell culture
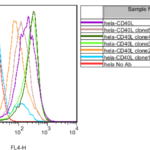
It has been proved that the activation of CD40 on B cells induced the rapid expansion of B cells in vitro using stable Fc receptor-expressing fibroblast cells as feeder cells and supplemented with anti-CD40 antibodies [20-22]. This CD40L-B system has been applied for the short-term culture of B cells in vitro. However whether the CD40L-B system could be used to improve EBV-induced transformation remains unclear. To answer this question, a new human CD40L (huCD40L)-expressing cell line was generated. CD40L gene was cloned from total RNA extracted from human PBMCs. Subsequently, cDNA was synthesized by reverse transcription from total RNA. Full-length CD40L was amplified by high-fidelity PCR and cloned into mammalian cell expression plasmid pcDNA3.1 (-). Clones were confirmed by sequencing. To generate stable CD40L-expressing Hela cells, Hela cells were transient transfected with confirmed plasmid. The expression of CD40L on Hela cells was confirmed by flow cytometry 2 days after transfection. Hela cells transfected with pcDNA: huCD40L were cultured for another 2 days before supplementation of G418. Two weeks after selection, G418-resistant clones were readily observed in a six-well plate. Six clones were selected for subculture in a 48-well plate and finally tested by flow cytometry. Five clones were found to be positive (Figure 5).
Figure 5
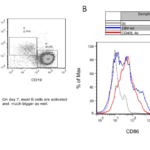
Figure 6 CD40L induced rapid B cell proliferation. (A) Proliferation of human B cells in the presence of H-CD40L was tracked on various time points by bright-field microscopy. (B) CD19+ B cells and CD3+ T cells were detected by flow cytometry on day 6. The ratios of B cells/T cells were quantified to compare B cell growth with or without stimulation on feeder cells. (C) B cell proliferation was measured by CFSE on day 6.
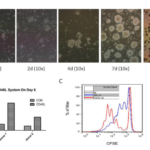
Given that a stable CD40-expressing cell line was generated, we examined whether it can activate the rapid expansion of human B cells in vitro. H-CD40L cells were irradiated at 80Gy before seeding in a six-well plate as feeder cells. The proliferation of B cells was assessed at various time points by bright field microscopy (Figure 6A). The results showed that B cells started to adhere on feeder cells on day 2. Clear cell clusters were observed on day 4. Cells expanded at a much faster speed since then. On day 7, a huge number of large cells formed in cell clusters could be observed. The cells were examined by flow cytometry on day 6. There was two- to four folds increase in B cells number compared with T cells (Figure 6B). The number of T cells was quite stable when CsA was supplemented in medium which could efficiently inhibit the activation and proliferation of T cells in vitro at 0.66μg/ml (Figure 8C). Finally, the proliferation of B cells was measured by CFSE on day 6. CD40L-B cells expanded much faster by achieving three to five rounds of cell divisions, whereas EBV-B cells only experienced one to two rounds of cell divisions (Figure 6C). These data proved that H-CD40L cells could be readily used as feeder cells for the rapid expansion of human B cells in vitro. EBV-transformed B cells could be easily detected by flow cytometry scatter analysis because of the change in cell size. Thus, we investigated if CD40L-activated B cells (CD40L-B) could be detected in a similar way. To this end, PBMCs were cultured in the presence of CD40L-expressing feeder cells for 7 days before flow cytometry analysis.
The lymphocytes were gated based on the difference in cell size. The large cells in the lymphoblast gate were exclusively B cells, whereas the small resting lymph cells were mainly T cells (Figure 7A). CD40L-B cells demonstrated a large cell size similar to EBV-B cells. So scatter analyze was used to detect CD40L-B cells as EBV-B cells. We further examined the activation of B cells in the CD40L system by focusing on the alteration in costimulatory molecules on B cells. CD86 was the commonly used marker for B cell activation as previously tested after EBV transformation, so the expression of CD86 was examined on day 4 after either CD40L stimulation or EBV transformation. B cells in the CD40L system experienced full activation compared with EBV-transformed B cells (Figure 7B). CD21 was the main EBV receptor on B cells with slight upregulation after EBV infection. The expression of CD21was examined on day 4 on CD40L-B and EBV-B cells. The results showed a similar upregulation of CD21 on CD40L-B and EBV-B cells. In conclusion, consistent with the rapid expansion of B cells in the CD40L system, B cells could be more efficiently activated by the CD40L system compared with EBV transformation. Besides, CD40L-B cells showed higher expression of costimulatory molecule CD86 than EBV-B. Similar to EBV-B cells, CD40L-B cells assumed an enlarged cell size, which could be readily detected by scatter analysis.
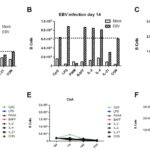
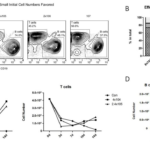
Given that H-CD40L could lead to rapid activation and proliferation of human B cells in vitro, we then asked whether H-CD40L can be used for the long-term culture of human B cells by comparing B cell growth in the CD40L system with EBV transformation. Freshly isolated PBMCs were first prepared from buffy coats and their components were quantified by flow cytometry. T cells accounted for over half (50%) in the whole population, followed by monocytes at 25%, whereas B cells accounted for only about 2.0%–5.0% in total PBMCs (Figure 8A). Then PBMCs were then seeded in six-well plates and cultured in the CD40L system and EBV transformation. The total numbers of cells were recorded every 4 days. Meanwhile, B cells and T cells were quantified by flow cytometry (Figure 8B). The proliferation of B cells in the whole lymphocytes (B/(T+B) ×100%) at various time points in the CD40L and EBV transformation systems is showed in (Figure 8C). Initially, B cells only accounted for 3.6% in the whole PBMC population. On day 4, the number of B cells was 4.3% after EBV transformation, whereas B cells turned to 9.8% in the CD40L system. The gap became increasingly significant since day 4. B cells accounted for 13.8% on day 8 after EBV transformation. By contrast, B cells increased sharply to 63.3% in the CD40L system at the same time point. However, B cells grew steadily in the EBV transformation system thereafter. The proliferation of B cells gradually slowed down as an increasing number of B cells started to die in the CD40L system (Figure 11). The growth curves of B and T cells in the CD40L and EBV transformation systems are showed in Figure 8D. About 1.3×, 4.1×, 13.4× and 27× increases in B cells were recorded on days 4, 8, 12 and 16 after EBV transformation, respectively. Meanwhile, about 3.5×, 40.2×, 84.9× and 211.3× fold increases were recorded on days 4, 8, 12 and 16 in the CD40L system. The number of T cells was quite stable with a slight reduction because of the presence of CsA in both the CD40L and EBV transformation systems. Hence, the ratio of B/T×100% could be roughly used to indicate B cell growth. Collectively, B cells proliferated in a relatively slow but steady manner after EBV transformation. By contrast, B cells proliferated much faster when cultured in the presence of CD40L-expressing feeder cells, especially between days 4 and 8.
Figure 7 CD40L induced fast and full activation of B cells. (A) PBMCs were cultured in the presence of CD40L-expressing feeder cells for 7 days before analysis by flow cytometry. Large lymphoblast cells were mainly B cells, whereas resting lymph cells were T cells. (B) Fast and full activation of B cells induced by the CD40L system.
Figure 8 Fast and early B cells expansion in the CD40L system than by EBV transformation. (A) PBMC components. (B) B cells growth in the CD40L and EBV transformation systems at different time points analyzed by flow cytometry. (C) B cells in total lymphocytes cultured in the two systems. (D) The growth curves of B and T cells in the two systems.
Effect of cell density on the efficiency of CD40-B cells
A high density of PBMCs (3-5×106 cells) was required for the conventional EBV transformation method because a high concentration can increase EBV transformation efficiency by facilitating the spread of virus via the close contact of cells. However, we found that B cells failed to proliferate with a high density of PBMCs in CD40L system. To determine the optional initial PBMC concentration for the CD40L system, PBMCs were seeded at 5×104/ml, 4×105/ml, 2×106/ml and 107/ml in a 6-well plate in the presence of CD40L-expressing feeder cells. The growth of B cells was assessed on day 10 by flow cytometry (Figure 9A). The results showed that B cells accounted for 73.3% and 73% when initial PBMC were seeded at 5×104/ml and 4×105/ml, respectively. However the proportions of B cells dropped to 48.1% and 37.5% when initial PBMC were set at 2×106/ml and 107/ml, respectively (Figure 9B). Thus, the optional initial PBMC concentration was 4×105/ml. Early experiments found that the presence of feeder cells causes B cell death after extended culture (Figure 8B and 8C). The main reason may be induced by the increasing number of irradiated feeder cells that died and detached from flasks after the extended culture time. Certain components released from dead cells may be detrimental to the viability and growth of CD40L-B cells. In addition, the pH of growth medium became acid in a shorter time when more irradiated feeder cells were seeded, possibly resulting in the death of CD40L-B cells. We then examined the optional density of feeder cells. To this end, irradiated feeder cells were seeded at 0, 4×104 and 2.4×105 in six-well plates. The growth of B and T cells was measured on days 3, 7, 10 and 14, respectively (Figure 9C). As expected, B cells did not grow in the absence of H-CD40L feeder cells. B cells proliferated equally well on feeder cells at 4×104/well and 2.4×105/well, so feeder cells at 4×104 /well in six-well plates were used in subsequent experiments. Taken together, the cell densities of both initial PBMC and irradiated feeder cells were critical for efficient B cells proliferation in the CD40L system. The optimal cell density for initial PBMCs and feeder cells were 4×105/ml and 4×104/well in a six-well plate, respectively.
Figure 9 Effect of cell density on B cell proliferation in the CD40L system. (A) Initial PBMC density determined B cell proliferation as tested on day 10 by flow cytometry. (B) The proportions of B and T cells on day 10. (C) Growth of B and T cells in the presence of different concentration of feeder cells in a six-well plate. (D) The presence of H-CD40 was required for the continuing culture of CD40L-B cells.
CD40 signal was indispensable for long-term survival of CD40L-B cells
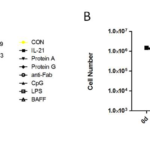
Previous data showed that the presence of H-CD40L cells is required but also detrimental for long-term culture of B cells. In this study, we investigated whether B cells can survive without the presence of feeder cells. PBMCs were isolated from three donors and cultured in the CD40L system for 16 days. Fresh feeder cells were replaced every 4 days. On day 16, feeder cells were withdrawn. Rapid expansion of B cells (300- to 400-fold increases) was observed in all three samples from days 4 to 16. However, CD40L-B cells ceased to proliferate after the withdrawal of feeder cells (Figure 9D). The data proved that CD40 signal was indispensable for the growth of CD40L-B cells. For the EBV transformation system, certain cytokines have been identified to enhance B cell immortalization efficiency, such as LPS, CpG and BAFF. We investigated whether the expansion of B cells in the CD40L system can be further improved by additional cytokines. To this end, IL-21, CpG, LPS, BAFF, protein A, protein G and anti-IgG F(ab)2 were tested in the CD40L system. The numbers of B and T cells were assessed on days 4, 9 and 14, respectively. B cells proliferated in a similar speed when treated with different cytokines (Figure 10A). These cytokines and activators showed no effects on T cells (Figure 10B). Thus, the commonly used cytokines could not enhance the proliferation of CD40L-B cells.
Figure 10 Effect of cytokines on outgrowth of CD40L-B cells. (A) B cell growth in the CD40L system stimulated with different cytokines or activators. (B) T cell growth in the CD40L system stimulated with different cytokines or activators.
EBV transformation rescued CD40L-B for long-term culture of B cells
Previous findings showed that irradiated allogeneic mononuclears cells can enhance EBV transformation. This method was successfully used to isolate SARS antibodies in 2004 [8] and autoreactive antibodies from patients with autoimmune disease in 2010 [18]. Costimulatory molecules on the allogeneic mononuclear cells have been suspected to be responsible for the activation of B cells. Given that CD40L was the strongest among these molecules, B cells experienced rapid expansion in the first few days in the CD40L system. However CD40L-B cells were unsuitable for long-term culture because of the poor viability of CD40L-B cells. By contrast, EBV-B cells proliferated in a slow but steady speed, which could be cultured in vitro nearly indefinitely. Subsequently, we investigated whether EBV immortalization efficacy can be further improved in the presence of CD40L feeder cells. First, we investigated whether EBV transformation rescues CD40L-B cells in vitro. Freshly isolated PBMCs were divided into four groups: one group served as blank control without any treatment. The other three were cultured with the EBV transformation, CD40L system and the two methods combined. Lymphoblastoid B cells were directly measured by flow cytometry at various time points (Figure 11). B cells did not proliferate in the control group as most lymphocytes were in resting status and no cell cluster formed on day 8 as check under microscope. By contrast, B cells were activated and proliferated steadily after EBV transformation. The formation of small cell clusters could be observed on day 8. In addition, B cells expanded rapidly in the CD40L system and peaked on day 8. Cell clusters were observed on day 8 with a much bigger size compared with EBV-B cells, but an increasing number of CD40L-B cells died. On day 20, most CD40L-B cells were already dead. Interestingly, for the new method that combined EBV transformation and CD40L stimulation, rapid expansion of B cells could be observed on day 8. In contrast to the CD40L system, B cells could long-term survive with similar cell viability as EBV-transformed B cells. These results proved that EBV transformation could rescue CD40L-B for long-term culture.
Evaluation of B cell immortalization efficiency by lymphoblastoid cell outgrowth
We evaluated B cell immortalization efficiency by lymphoblastoid cell outgrowth assay. Freshly isolated PBMCs were first incubated with concentrated EBV by spinfection methods before seeding into wells of 96-well round bottom plates as a fivefold dilution series. Cells were incubated for 21 days in a tissue culture incubator at 37 °C and 5% CO2. Cells were checked on day 21 as the outgrowth of lymphoblastoid cell was visible under microscopy by then (Table 3). The results showed that the efficiency of the methods was approximately 7.8% (0.6%–20%), which was slightly higher than that of the improved EBV transformation method of 2.4% (0.4%–11%). The variances among PBMCs were quite obvious.
Figure 11 EBV transformation rescued CD40L-B for long-term culture. Freshly isolated PBMCs were divided into four groups: blank control, EBV transformation, CD40L system and EBV/CD40L method. Lymphoblastoid B cells were measured by flow cytometry on days 4, 8, 12 and 16.
Generation of immortalized human naïve B cell libraries based on improved EBV immortalization method.
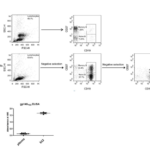
Early naïve B cells were usually non-memory B cells that assumed the phenotype as CD19+IgM+CD27-. Accordingly, B cells with the same phenotype were isolated by magnetic beads (Miltenyi) in a two-step negative selection method. In brief, non-B cells were indirectly magnetically labeled and deleted by human B cell isolation kit. Memory B cells were directly deleted with CD27 beads. The purity of the purified B cells and naïve B cells were stained with anti-CD19-APC and anti-CD27-FICT and then analyzed by flow cytometry. The results showed that high-purity of B and naïve B cells were isolated after the first and second rounds of negative isolation, respectively (Figure 12). The size of an immortalized B cells library is generally determined by both the initial number of B cells and the efficiency of B cell immortalization efficiency. The efficiency of B cell immortalization improved to 7.8% by the optimized EBV transformation method. Given that the number of starting naïve B cells also limited the diversity or the size of B cell libraries, human naïve B cells were isolated from 16 healthy donors and immortalized by the optimized EBV method. Finally, an immortalized human naïve B cell library was generated by combining all the individual libraries together. The size of the new library was estimated to be about 6×106. The plasma of some donors (9/16) was examined by gp140YU2-ELISA. HIV-1 neutralizing antibody b12 was used as the positive control at 5 ng/ml. As suspected, the plasma could not recognize the HIV-1 envelope as they were derived from health donors (Figure 12). This library is aimed for isolation for non-HIV-1 binding antibody ancestor expressing naïve B cells. Therefore, an immortalized human naïve B cell library with the diversity of BCR repertoire at about 6×106 was generated from health donors.
Figure 12 Generation of immortalized human naïve B cell libraries. (A) Purification of human naïve B cells from PBMCs of healthy donors. PBMCs, purified B cells and purified naïve B cells were stained with CD27-FITC and CD19-APC, and then analyzed by flow cytometry. (B) The plasma could not bind gp140YU2 as tested by gp140YU2-ELISA.
Discussion
Strategies other than EBV transformation have also been documented for human B cell immortalization. For example, human B cell hybridoma technology has been developed to isolate GM-CSF specific antibody. However, its efficiency (<10-6) is much lower than the conventional EBV method (10−6–10−4) [23]. In addition, a genetic programming method for the generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells has been reported by the exogenous expression of Bcl-6 and Bcl-xL in GC B cells [24]. A RSV-specific B cell line was generated by this method. However, this method is technically demanding with comparatively low efficiency. Thus, EBV transformation is still the first choice for the immortalization of human B cells.
In this study, different approaches were investigated to improve EBV transformation efficiency for generating immortalized human B cell libraries with enlarged cell diversity. The infection of human B cells with EBV usually led to the expression of latency III genes, which was characterized by the emergence of LMP1, LMP2 and EBNA1-6. The expression of these genes was proven to be responsible for the latency and immortalization of B cells after EBV transformation [2]. However the immortalization efficiency for conventional EBV transformation was about 10−6–10−4, which was too low to maintain the diversity of the B cell repertoire, indicating that the size of the B library was only about 102–104 starting from 108 primary B cells. Hence, great efforts have been made to improve the immortalization efficiency of primary human B cells as followed.
The activation and proliferation of B cells were correlated with the titer of EBV stocks. Since the B95.8 cell line was the main resource for preparing EBV stocks and viral titers were usually quite low, increasing the titration of EBV titer was critical. We found that TPA could significantly induce the release of EBV and increase the titer of EBV stocks. When combined with 10× concentration of EBV-containing supernatant, we observed a four- to eightfold increase in the activation and proliferation of B cells. In contrast to previous reports that EBV titrations are measured by quantitative real-time PCR, transforming units/ml was used in our study because it could better recapitalize EBV transformation ability. We found that the proliferation of LCLs was logarithmically dependent on the EBV dose. By this method, the titer of EBV could reach 107 U/ml which was sufficient for further experiments. It was noted that transformation efficiency was positively correlated with the EBV titer, but not in a linear relationships. Previous findings showed that B cells with multiple EBV infection events do not provide additional immortalization advantage than a single infection event, suggesting that when EBV titer increased to a level that could have most B cells infected, further improvement in viral titer might not bring additional benefits.
The infection efficiency of envelope viruses could be improved by low-speed centrifugation in the first few hours of infection. Given that spinfection in previous studies varied and only short-term infection efficiency was examined, we investigated the optimal spinfecion conditions for long-term B cells immortalization. EBV transformation efficiency significantly improved by spinfection. Interestingly, we found that the short-term EBV transformation efficiencies as measured on day 3 increased in a similar level by indifferent centrifugal conditions, including different centrifugal forces, times and temperatures. However, in terms of long-term immortalization of B cells, centrifugation at 500 g for 30min–60mins produced optimal results, and temperature had no influence on B cell immortalization.
Difference cytokines and B cell activators have been evaluated for EBV-induced B cells transformation. Unlike previous reports that measured short-term activation and proliferation of B cells (3–6 days), long-term B cell proliferation was investigated in this study (21 days). Consistent with previous findings, CpG was the most potent activator for short-term B cell proliferation [4]. However, BAFF was found to be the most important cytokine for long-term proliferation of B cells. Finally, the efficiency of B cell immortalization was assessed by lymphoblastoid cell outgrowth assay. The immortalization efficiency was approximately 2.4% (0.4%–11%), which was much higher than convention EBV transformation methods (10-6-10-4). The diversity of the B cells library was 2.4×106 starting from 108 primary B cells.
CD40, a member of the TNF receptor family, was expressed on mature B cells and could be activated by its ligand, CD40 ligand (CD40L), which was mainly expressed on activated T cells. The interaction of CD40L with CD40 provided the most important second signal for the activation and proliferation of B cells [25]. Patients with CD40L deficiency usually suffer from hyper-IgM syndrome manifested by a lack of T cell mediated B cell response in vivo [26]. GC structure, memory B cells, CSR and SHM could not be found in such patients [27]. CD40L was identified as the most important costimulatory molecule for B cell activation and proliferation, so it was not surprising to find that CD40L-expressing fibroblast could be used as feeder cells and induce the rapid expansion of human B cells in vitro than EBV transformation [22]. This CD40L-B system has been used for short-term culture of human PBMCs to obtain highly purified B cells, which showed great therapeutic value for cancers and infectious diseases using as antigen-presenting cells [12]. However, whether the CD40L system can be used for long-term B cell culture and whether it can synergistically enhance EBV transformation remain unknown. Accordingly, we generated a new human CD40L-expressing cell line, termed H-CD40L. Consistent with previous documents, rapid activation and expansion of human primary B cells were observed. High-purity human B cells could be harvested by the CD40L system with proper modifications of culture conditions. The proliferation of B cells was completely dependent on the presence of CD40L feeder cells. Meanwhile, the viability of B cells was greatly impaired because of the detachment of feeder cells and release of toxic components in the medium. Thus, an increasing number of B cells started to die since week 2, which made the long-term culture of CD40L-B cells quite difficult, if not impossible. The EBV transformation efficiency was investigated in the presence of CD40L-expressing feeder cells. Interestingly, enhanced EBV transformation efficiency was observed in the presence of feeder cells. The results showed that the efficiency of this method was 7.8% (1%–20%), which was 3 folds higher than that of the optimized EBV transformation method at 2.4% (0.4%–11%). Nevertheless, the viability of EBV-transformed B cells in the presence of feeder cells was still poor.
By comparing the EBV transformation efficiencies in distinct subsets of B cells, we found that lower EBV transformation efficiency in memory B cells was paralleled with the reduced rate of their proliferation compared with naïve B cells [19]. The rapid proliferation of B cells in the CD40L system may be responsible for the increase in EBV transformation efficiency. EBV-induced transformation was usually termed EBV immortalization. However, EBV transformed B cells are actually mortal especially in the early stage manifested by normal diploid karyotype and low telomerase activity [7]. Besides, EBV transformation efficiencies varied greatly with the PBMCs isolated from different donors. Moreover, the viability and transformation efficiency would greatly be reduced when PBMCs or B cells were revived from −150 °C stocks compared with freshly isolated ones. In conclusion, the immortalization efficiency of human primary B cells was at around 7.8%, which made it possible to generate immortalized human B cell libraries for the isolation of HIV-1-specific B cells. An immortalized human naïve B cell library with BCR repertoire diversity at about 6×106 has been generated from healthy donors.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
We wish to thank Prof. G.S.W. Tsao (Department of Anatomy, Li Ka Shing Faculty of Medicine, HKU) for providing EBV-producing marmoset B cell line (B95-8). We thank Zhiwu Tan, Zhiwei Chen (AIDS Institute, HKU) for helpful discussions. This work was supported by Hong Kong Health and Medical Research Fund (HMRF) (# 11101052) and General Research Fund (GRF) (# 785112), and China 12th 5-year Mega project for HIV/AIDS (# 2012ZX10001006) to M-Y. Z.
References
- Rosen, A., et al., Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature, 1977. 267(5606): p. 52-4.
- Knipe, D.M. and P.M. Howley, Fields Virology 6th Edition. 2013.
- Sun, Z., et al., Isolation and characterization of HIV-1 envelope glycoprotein specific B cell from immortalized human naive B cell library. J Gen Virol, 2017.
- Iskra, S., et al., Toll-like receptor agonists synergistically increase proliferation and activation of B cells by epstein-barr virus. J Virol, 2010. 84(7): p. 3612-23.
- Gires, O., et al., Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J, 1997. 16(20): p. 6131-40.
- Caldwell, R.G., et al., Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity, 1998. 9(3): p. 405-11.
- Sugimoto, M., et al., Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res, 2004. 64(10): p. 3361-4.
- Traggiai, E., et al., An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med, 2004. 10(8): p. 871-5.
- Walker, L.M., et al., Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature, 2011. 477(7365): p. 466-70.
- Macagno, A., et al., Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol, 2010. 84(2): p. 1005-13.
- Yu, X., et al., An optimized electrofusion-based protocol for generating virus-specific human monoclonal antibodies. J Immunol Methods, 2008. 336(2): p. 142-51.
- Schultze, J.L., et al., CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest, 1997. 100(11): p. 2757-65.
- Nojima, T., et al., In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat Commun, 2011. 2: p. 465.
- Walker, L.M., et al., Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science, 2009. 326(5950): p. 285-9.
- Falkowska, E., et al., Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity, 2014. 40(5): p. 657-68.
- Sun, Z., et al., Reconstitution and characterization of antibody repertoires of HIV-1-infected “elite neutralizers”. Antiviral Res, 2015. 118: p. 1-9.
- Yang, Z., et al., Identification of Non-HIV Immunogens That Bind to Germline b12 Predecessors and Prime for Elicitation of Cross-clade Neutralizing HIV-1 Antibodies. PLoS One, 2015. 10(5): p. e0126428.
- Fraussen, J., et al., A novel method for making human monoclonal antibodies. J Autoimmun, 2010. 35(2): p. 130-4.
- Dorner, M., et al., Distinct ex vivo susceptibility of B-cell subsets to epstein-barr virus infection according to differentiation status and tissue origin. J Virol, 2008. 82(9): p. 4400-12.
- Andersson, J., et al., Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell, 1977. 10(1): p. 27-34.
- Banchereau, J. and F. Rousset, Growing human B lymphocytes in the CD40 system. Nature, 1991. 353(6345): p. 678-9.
- Arpin, C., et al., Generation of memory B cells and plasma cells in vitro. Science, 1995. 268(5211): p. 720-2.
- Li, J., et al., Human antibodies for immunotherapy development generated via a human B cell hybridoma technology. Proc Natl Acad Sci U S A, 2006. 103(10): p. 3557-62.
- Kwakkenbos, M.J., et al., Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med, 2010. 16(1): p. 123-8.
- van Kooten, C. and J. Banchereau, CD40-CD40 ligand. J Leukoc Biol, 2000. 67(1): p. 2-17.
- DiSanto, J.P., et al., CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature, 1993. 361(6412): p. 541-3.
- Xu, J., et al., Mice deficient for the CD40 ligand. Immunity, 1994. 1(5): p. 423-31.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/