J Med Discov (2017); 2(1):jmd17002; doi:10.24262/jmd.2.1.17002; Received February 23rd, Revised March 6th, Accepted March 6th, Published March 12th.
Flu Pathogenesis Proteolytic Theory and its Role in the Improvement of Flu’s Treatment
Valentina A. Divocha1,*, Yroslav Basarab2
1Ukraine Scientific Research Institute of Transport Medicine , Odessa 65039, UA
2Ukrainian Academy of Medical Dental, Poltava, UA
* Correspondence: Valentina A. Divocha, Experimental and Clinical Pathology Laboratory, Ukraine Scientific-Research Institute Of Transport Medicine, 92, Kanatnaya St., Odessa 65039, UA. Email: divocha09@ukr.net, Tel/Fax: 048 728 14 52
Abstract
A new theory of grippe pathogenesis with the use of proteinase-inhibitory system has been offered. It has been established that purification and concentration of grippe viruses by different methods did not release the virus from the cellular enzymes. When the experimental animals have been infected with grippe virus, we have observed misbalance in enzyme-inhibitory balance took place, especially during first hours after infection. Six isoforms of trypsin-like proteinases were obtained from the lungs of healthy mice. Antiproteinase immune sera have been isolated from these 6 isoforms which served to treat the experimental animals. It was antiserum to the third isoform that has prevented the experimental animals mortality. From the industrial wastes of gamma-globin manufacture the inhibitors of protein-like proteinases have been extracted which prevented the white mice’s mortality in 80% of cases. Endogenous inhibitors of human blood proteinases are prospective in producing anti-influenza drugs for humans.
Keywords: flu virus, tripsin-like proteinase, inhibitor of proteinases
Introduction
In the pathogenesis of viral diseases the interaction of virus with the cell has not been sufficiently studied. The main point here is the virus intervention into a healthy cell with a mandatory deproteinization of the virus. However, deproteinization of viruses is still poorly understood. This firstly refers to the mechanism of influenza virus introducing into cells of mammals, including humans. In this regard, in 1983 we proposed a new theory of influenza pathogenesis with proteinase-inhibitory system participation [1, 2].
The difficulties in creating products with selective antiviral action are connected with biology of viral diseases pathogens. Recent achievements of biochemistry and molecular biology, revealing the features of virus reproduction, help to create new generations of drugs with directed intervention in the cycle of viral reproduction [3-6].
The purpose of the study is to examine the state and role of antiproteinase systems of virus and recipient in the development of influenza infection to obtain and use fundamentally new therapeutic drugs based on inhibitors of trypsin-like proteases.
The Research objectives in this study include the follow six parts. The first one is to clear flu virus till homogenous state. The second one is to explore the nature of protease associated with influenza virus. The third one is to examine the role of proteases and their inhibitors in different, especially at early stages, of influenza infection development. The fourth one is to isolated and purified proteinase and its inhibitor from the lungs of healthy mice as well as from those infected with flu virus. The fifth one is to obtain specific antibodies to isoforms of trypsin-like proteases and study their protective effect under the conditionts of experimental influenza. The last one is to study the protective effect of cellular inhibitors on infection of animals with a lethal dose of influenza virus.
Materials and Methods
Materials
Strains of influenza virus: A/PR/8/34 (H1N1), A/Aichi/2/68 (H3N2), A/USSR/90/77 (H1N1), A (Extra X-31), A/WSN/33 (H1N1), A/Phil/2/82 (H3N2), AO/32 (NON1) grown on a 9-day chicken embryos, B strain PR-109, obtained by recombination of viruses V/Lee/40 and B / USSR / 100/83, and MDCK cells were obtained at the I.D. Ivanovsky Research Institute of Virology, Academy of Medical Sciences of Russia and strain AO/32 (HON1) – from the Influenza Research Institute of St. Petersburg, Russia. The other materials used in this work include 2358 items of white mice, line BALB/c and hybrids; 3052 pcs of chicken embryos; 1612 tubes of passaged MDCK cell culture;140 items of white rats, Wistar line.The virological, biochemical, immunological, molecular biological, radioisotopic techniques were used in this study.
Proteolytic activity detection
To study the nature of proteolytic activity associated with influenza virus we used 10-11-day chicken embryos and influenza virus AO/32 (HON1) with infectious titers 7 lg EID 50 / 0,2 and HA-1: 256. The virus was accumulated by infecting chicken embryos in a volume of 0.2 ml, diluted to 10-3 with infectious material. Infected chicken embryos were incubated for 48 hours at +36 ° C. Then they were cooled for 18 hours at +4 ° C and then the virus-containing fluid was collected.
Results and Discussion
In the early 80’s, in purifying and concentrating of various strains of influenza virus to produce polyvalent anti flu vaccines, we faced the fact that we could not release the influenza virus from proteolytic activity (Fig. 1) [7]. To resolve this problem, we have improved the methods of purification, but nevertheless failed to release influenza virus from proteolytic activity.
We analysed purified preparations of influenza virus for the presence of proteolytic activity and revealed that the treatment of influenza virus by ultracentrifugation techniques does not relieve flu virus from proteolytic activity. In saccharose gradient (15-60%) proteolytic activity was clearly separated into several isoforms (Tab. 1) [8].
The results obtained allowed us to conclude that flu virus is associated with serin-containing protease of trypsin –like type of cell origin, which has molecular heterogeneity. Our further studies (Tab. 2) showed that all commercial products contained both inhibitor, and trypsin-like protease, i.e. the today’s drugs are not cleared completely of protein impurities, or it is impossible to separate the viral proteins from cellular components. Viral proteins are tightly associated with components of the cell, so the structure of influenza virus should be considered taking into account the interaction with cellular enzymes and their inhibitors.
In the body the system of proteinases and inhibitors is represented by a large group of proteins. Inhibitors of proteolytic enzymes counterbalance the corresponding enzymes in the body, and are in constant dynamic equilibrium with them [9-12]. Violation between enzymes and inhibitors is important for the development of pathological processes [13].
Our studies show that in the lungs and blood serum of uninfected animals and chicken embryos, the level of protease activity and protease inhibiting activity are in equilibrium, and the latter is violated at influenza virus A infection.
In the infectious process the most profound changes occur in the first hours after infection. So, in 6 hours after infection the number of proteinases in the lungs and serum of infected animals is reduced and inhibitory activity is increased [14]. Influenza virus infected cells induce the appearance of the inhibitor both in the lung tissue and blood serum. Consequently, lung inhibitors are like the first line of an organ defense under the action of various strains of influenza virus.
Figure 1. Scheme of purification of influenza virus.
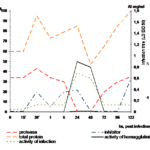
When studying the dynamics of proteinase and inhibitory activity in the chicken embryos under the influence of large and small infecting doses of influenza virus A/RR/8/34 we have found that there occurred the same changes as in white mice organisms (Fig. 2). During the period of maximal accumulation of infectious and hemagglutinating activity (24 hours) they did not find either proteinase or inhibitory activity [15].
Six isoforms of trypsin-like proteinase have been isolated from the lungs of healthy mice by while 8 isoforms have been isolated from the lungs of infected mice. Their specific proteolytic activity increased sharply compared with the initial material (Tab. 2). Proteinase obtained isoforms had a broad substrate specificity and were capable to hydrolyze both natural and synthetic substrates [16]. Antiproteinase hyperimmune rat sera have been obtained to all isoforms of trypsin-like proteinases. When studying the protective properties of antiproteinase sera and sera of healthy rats on white mice infected intarnasally with lethal dose of influenza virus A/PR/8/34 (IV passage), it was found that 100% mortality of control mice took place in 4-5 days (Tab. 3). The animals to which they administrated six times the healthy rats serum intranasally died on the 7th day. In the treatment of mice with the pools of immune sera I, II, IV, V and VI the animals mortality rate was reduced and lethality came much later than in the control group and 20% of the animals recovered [17].
Table 1 Purification of influenza virus АО/32 in succharose gradient and ultracentrifugation at 28000 min.-1, 4 h
| Fractions of sacchrose gradient | Experiments | |||||||||||
| 1 VCF * | 2 VCF * | 3 VCF * | 4 OAF ** | |||||||||
| % saccharose | HR1 | proteinase, mg/ml | % saccharose | HR1 | proteinase, mg/ml | % saccharose | HR1 | proteinase, mg/ml | % saccharose | HR1 | proteinase, mg/ml | |
| 1 | 5 | 0 | 1,6 | 3 | 0 | 1,5 | 6 | 0 | 2,6 | 6 | 0 | 0,58 |
| 2 | 15 | 1:8 | 36 | 11 | 1:2 | 13,6 | 15 | 1:8 | 41,3 | 17 | 0 | 1,01 |
| 3 | 32 | 1:16 | 9,6 | 24 | 1:16 | 8,2 | 23 | 1:16 | 0,45 | 27 | 0 | 0,37 |
| 4 | 42 | 1:2048 | 33,6 | 24 | 1:16 | 10,2 | 30 | 1:64 | 0,9 | 29 | 0 | 0,59 |
| 5 | 49 | 1:2048 | 4,4 | 38 | 1:512 | 25,4 | 37 | 1:512 | 13,8 | 37 | 0 | 0,80 |
| 6 | 52 | 1:64 | 37 | 41,5 | 1:1024 | 26,2 | 44 | 1:16000 | 38 | 41 | 0 | 1,43 |
| 7 | 55 | 1:64 | 37,4 | 46,5 | 1:512 | 12,2 | 47 | 1:16000 | 3,29 | 49 | 0 | 0,64 |
| 8 | 57 | 1:62 | 6,8 | 53 | 1:16 | 7,9 | 51 | 1:256 | 0,3 | 53,5 | 0 | 1,28 |
| 9 | 57 | 1:512 | 56 | 0 | 1,08 | |||||||
Notes: * virus-containing fluid;
** original allantoic fluid; 1 – hemagglutination reaction
Table 2 Purification DEAE –cellulose-53 of lung trypsin-like proteinase of healthy mice
| N of fraction | N of isoform | Specific proteolic activity per mg of protein | % of proteinase outcome | % of purification by protein |
| 33 | I | 4,285 | 2,09 | 96,8 |
| 53 | II | 83,75 | 5,84 | 99,07 |
| 65 | III | 22,42 | 2,703 | 98,38 |
| 75 | IV | 40,00 | 6,279 | 97,92 |
| 121-130 | V | 32,6 | 136,74 | 99,98 |
| 161-189 | VI | 0,787 | 421,74 | 64,90 |
The most effective was the fourth pool of imunne serum to the IΙΙ isoform. In its presence 60% of infected mace survived, and on the 14th post- infection day in the blood serum and in the lungs, we did not detect either hemagglutinin, no infectious virus. Imunne serum to isoform VI did not protect mice from death, although isoform IΙΙ differed from isoform VI by only one protein with molecular mass 32 kDa [18].
Table 3 Influence of antiproteinase immune sera on survival of mice under infection with lethal dose of influenza virus A/PR/8/34
|
N Gr |
Isoform of proteinase |
Sera group | Terms after infecting , days | |||||||||||||
|
6 h |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
14 |
survived | % of survive | |||
| 1 | I | I | 2/10 | 2/10 | 2/10 | 2/10 | 2 | 20 | ||||||||
| 2 | I | II | 2/10 | 2/10 | 4/10 | 2 | 20 | |||||||||
| 3 | II | III | 2/10 | 2/10 | 2/10 | 2/10 | 2 | 20 | ||||||||
| 4 | III | IV | 2/10 | 2/10 | 6 | 60 | ||||||||||
| 5 | IV | V | 2/10 | 2/10 | 4/10 | 2 | 20 | |||||||||
| 6 | V | VI | 5/10 | 3/10 | 2/10 | 0 | 0 | |||||||||
| 7 | VI | VII | 7/10 | 1/10 | 1/10 | 1 | 10 | |||||||||
| 6 | Sodium chloride solution | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 10 | 100 | |
| 7 | Serum-free virus | 2/10 | 2/10 | 6/10 | All
died
|
0 | ||||||||||
| 8 | Healthy rats serum | 2/10 | 3/10 | 5/10 | All
died
|
0 | ||||||||||
| 9 | Immune serum IVgr., virus-free (toxicity) | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 10 | 100 | |
Note: 1. numerator – number of mice dies; 2 – denominator – number of mice in the experiment.
From the lungs of healthy mice, we have isolated an inhibitor of trypsin-like proteases with molecular mass of 47.5 kDa, with a high degree of purity and small amount of impurities. We have developed and patented the method of obtaining and purification of trypsin proteinase inhibitor [19]. The inhibitor isolated is similar to the α1-proteinase inhibitor of human blood serum (48-55 kDa) and egg white trypsin inhibitor (49 kDa), but does not have not likeness to trypsin inhibitor isolated from the lungs of cattle (an inhibitor of Northrop-Kunitz type), which had a molecular mass of 65 kDa. In the study of its effect on the proteolytic activity of trypsin-like proteinase isoforms by the test-tube method it has been shown that it inhibited the activity of almost all isoforms, except isoforms IV (41,8%) and VIII (28.3%). In our studies [20] we used a cellular inhibitor to suppress the development of influenza virus in chicken embryos. It has been revealed that it inhibited the development of infectious and hemagglutinating activity and the formation of total protein. At the same time, the inhibitor of trypsin-like proteinase isolated from the lungs of mice, previously infected with influenza virus, did not have this ability. In the further studies [21] for the treatment of influenza infection in animals, we used the inhibitor, which was isolated from the lungs of healthy mice.
Figure 2 Changes of proteinase and inhibiting activity in chicken embryos at a big infecting dose of influenza virus A/PR/8/34. AI– 1 unit equals 1 mg crystalline trypsin, A– 1 unit activity equals 1 mkg arginin / min.
The introduction of this inhibitor to mice previously infected with a lethal dose of influenza virus reduced the death rate from this disease due to inhibition of HA splitting on reproduction of the virus in the lungs, stopping the generalization of the process, preventing the increase of proteolysis in the lungs, as well as preventing aerohematic barrier and improved some of the reactions of local protection.
For an antiviral drug preparation that has the lowest allergic action on humans, we used wastes of donor blood which is taken for isolation of gamma – globulin and albumin.
At the first stage of γ-globulin and albumin manufacture from fraction (II +III, by Cohn E.J. ) fibrinogen is precipitated, which is later recycled. According to our data, the wastes contain 481.11 mg of trypsin-like proteinase inhibitor per kg of weight. This centrifugate contained α1 – antitrypsin, which is the main inhibitor of serine proteases of human blood plasma. Its share in the norm is 90% of antitripsin activity of human blood plasma [22].
In the second step of γ-globulin obtaining, precipitate containing prothrombin, α-and β-globulins and lipoids is utilized. This precipitate, according to our data [23], contains 469.87 mg of trypsin proteinase inhibitor per kg of mass. Antithrombin-3 (AT-3) or heparin factor – a regulator of blood coagulation is included in this sediment. According to O.A Markova et al. [24], normally the content of AT-3 in donors ranged from 160 to 250 mcgr / ml. α1-antitrypsin and α2-macroglobulin [24, 25] are also in the area of α-globulins [24, 25].
Table 4 Action of cellular inhibitor of trypsin-like proteinase of the survival rate of mice infected with lethal dose of influenza virus А/PR/8/34
| N and name of
group |
Number of animals | Dose of influenza virus | Dose of inhibitor on mice per protein | Number of animals | % of animals protected from virus | |
| Dies | Survived | |||||
| 1. Influenza virus | 40 | 10-3 | – | 40 | – | 0 |
| 2. Influenza virus +trypsin, crystal. | 40 | 10-3 | 18 mkg | 40 | – | 0 |
| 3. Influenza virus + inhibitor from healthy lungs | 40 | 10-3 | 18 mkg | 7 | 33 | 82,5 |
| 4. Cellular inhibitor | 40 | 10-3 | 18 mkg | – | 40 | 100 |
| 5. Trypsin, crystal | 10 | – | 18 mkg | – | 10 | 100 |
| 6.Phosphate buffer | 10
|
– | 0,2 ml | – | 10
|
100
|
In the third stage of γ-globulin obtaining the sediment containing plasminogen goes to waste. At this stage wastes, according to our data, the content of trypsin proteinase inhibitor was 137.40 mg/kg.
At the fourth stage at γ-globulin sedimentation centrifugate N 3 is utilized. According to our records, the material of centrifuged № 3 contains 166.37 mg of trypsin – like proteinase inhibitor.
Thus, the waste product of the first and second stages of the technological process in which sediment (II +III) is washing and the allocation of prothrombin takes place may serve as the raw material for producing an inhibitor of trypsin-like proteinases. These wastes contained the highest number of trypsin-like proteinase inhibitor.
To separate trypsin-like proteinase inhibitor we used the wastes of the Ith stage (II + III) of γ – globulin receipt from donor blood, which contained a significant amount of this inhibitor. We isolated trypsin-like proteinase inhibitor from the centrifugate (wastes) of fraction (II + III) of the first stage of the γ-globulin by ion-exchange chromatography on DEAE-cellulose-53 (Watman, USA).
This method allowed to obtain five isoforms with inhibitory activity. The first two isoforms, in which there was a high content of trypsin-like proteinase inhibitors, have been eluted from ion-exchange column with 0.1 M phosphate buffer, pH 7.5. The next three isoforms containing trypsin-like proteinase inhibitor have been eluted by stepwise NaCl gradient of different molarity: the third isoform – 0.1 M NaCl, the fourth isoform – 0.2 M NaCl, the fifth isoform – 0.5 M NaCl. The volumes of isoforms eluates were, respectively: Ist – 35 ml, IInd-195ml, IIIrd – 340 ml, IVth – 440 ml, Vth – 605ml.
The highest content of trypsin-like proteinase inhibitor has been registered in the fractions of the Vth isoform, which was the last eluted from the column 0.5 M NaCl, while the lowest content was in the IVth and IIInd isoforms eluted from the column 0,2 and 0,1 M NaCl, respectively.
Trypsin-like proteinase plays a key role in the development of pathological process in an organism. It splits the outer protein of the influenza virus – hemagglutinin into two subunits: HA1 and HA2. Only after the splitting of hemagglutinin with this protease, the virus enters the cell and begins to multiply. Inhibitors block the process of cleavage of viral proteins by inhibiting the activity of cellular enzymes.
The viral progeny with non-split, functionally non-active viral proteins is formed in the presence of inhibitors of cellular trypsin-like proteinases after a single reproduction cycle of the primary virus with split proteins. Subsidiaries virions are not able to initiate the infectious process because of the block of the early stages of the reproduction cycle – adsorption and penetration of the virus [26, 27].
To study the protective action of trypsin-like proteinase inhibitor on the survival of mice infected with the lethal dose of influenza virus A/PR/8/34, we took 90 white mice of BALB/c line weighting 16-18 g and the fifth isoform of proteinase inhibitor isolated from the wastes of the first stage of γ- globulin production, as it had the highest indexes of the inhibitor activity (132.52 g / l) and the lowest indexes of trypsin-like proteinase (0.0027 mmol in the sample).
The mice were divided into 7 groups containing 15 items in each, with 10 items in control groups (Tab. 4). The animals of the first group received lethal dose of virus (virus control). The virus was administered intranasally in a volume of 0.05 ml under Rausch anesthesia. The second group of animals got a similar dose of virus, but they were treated with crystalline trypsin (control of medical properties of crystalline trypsin) in the same doses and terms as the animals of the 3rd group.
The third group of animals was infected with the same dose of virus and was treated with trypsin – like proteinase inhibitor obtained from the wastes of γ-globulin.
The fourth group of animals got only an inhibitor of proteinases from the wastes of γ-globulin production (control inhibitor’s toxicity).
Crystalline trypsin (trypsin control) alone was introduced to the animals of the fifth group, the sixth group got phosphate buffer, with which we diluted virus inhibitor and trypsin. The seventh group was used as the control of intact animals. Both trypsin and inhibitor was administered intranasally under light ether anesthesia during seven days. Each mouse got up to 140 mcg of the inhibitor for the course of treatment.
The results of our studies showed that the animals of the first and second groups died on the 6-7 post-infection day. In the third group 12 mice survived (80%). They remained alive for 14 days after infection (observation period). Animals of the 4th, 5th, 6th and 7th groups remained alive throughout the whole period of observation. In addition, newly acquired inhibitor of trypsin-like proteinases, did not cause toxicity, as white mice of the 4th group remained alive at the 14th day after introducing of the inhibitor.
Thus, the findings of our work indicate that trypsin-like proteinase inhibitor obtained from the wastes of the first stage of γ – globulin production possessed antiviral properties. It is not excluded that it can be used not only for influenza treatment but for the treatment of other viral infections in which the splitting of viral precursor protein is produced by cellular tripsin-like proteinases [21].
Conclusion
One of the most important stages of the development of many viruses in the host organism is their introduction into the cell after preliminary deproteinization. Regulation of this cycle by proteolytic enzymes of the host-cell is one of the fundamental principles of viral reproduction. Induction or introduction of inhibitor of the virus proteolytic activation is one of the promising ways of viral diseases treatment, including influenza.
Inhibitor of trypsin-like proteinases obtained from industrial wastes of γ-globulin manufacture blocked the development of grippe infection in white mice preliminary infected with lethal dose of influenza virus A/PR/8/34 (A/H1N1).
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
None
References
1. Divocha VA, Mikhalchuk VN, Gozhenko AI. Molecular-and-biological substantiation of antiproteinase therapy of flu. J.Acad.Med.Scien.Ukraine. 15, 1, 19-21 (2009) [Rus]Divocha VA, Mikhalchuk VN, Gozhenko AI et al. Trypsin-like proteinase and its inhibitors in vaccines and immunobiological preparations of blood. J.Acad. Med. Scien.15, 3, 609-25 (2009) [Rus]
2. Divocha VA, Mikhalchuk VN, Gozhenko AI et al. Trypsin-like proteinase and its inhibitors in vaccines and immunobiological preparations of blood. J.Acad. Med. Scien.15, 3, 609-25 (2009) [Rus]
3. Boortseva YeN, Shevchenko YeS, Leniova IA et al. Sensiitivity to remantadin and arbidol of flu viruses caused epidemics in Russia in 2004-2005. Problems Virology. 52, 2,24-9 (2007) [Rus]
4. Derebin PG, Kaplina EN, Nosick DN et al. Antiviral activity of ferrovit and derinap as to infection caused by pathogenic variant of bird flu A (H5N1).Med Chair,1,62-5 (2007) [Rus]
5. Savinova OV, Pavlova NI, Boreko YeI. Individual and complex use of new derivatives of betulin and remantadin for inhibition flu virus reproduction // Modern Problems of Humans Infectious Pathology: Collected Scientific Works.- Minsk (Belorussia): BELPRINT, 1, 137-41 (2008) [Rus]
6. Shevchenko Yes, Boortseva Y, Ivanova N et al. Specific antiflu chemical preparations, substantiation of their use for prophylaxis and treatment in Russia: Urgent Problems of Infectious Pathology and Vaccine Prophylaxis in Children, 13-14 December, 2007, Moscow: Abstracts.36-8 (2007) [Rus]
7. Divocha VA, Degtiarenko VI, Zevakov VF. Cellular Proteinase of Flu Virus: The 2nd Congress of Infectionists of Ukraine: Abstracts.- Kiev, 36-8 (1983) [Rus]
8. Divocha VO Studing of Proteolytic Activity during purification of flu virus by centrifugation. Odessa Med J.,1, 16-9 (2007) [Ukr]
9. Zhdanov VM, Gaydamovich SYa. Family of Orthomyxoviridae. Partic. Virol: Manual, Moscow, 2, 139-85 (1983) [Rus]
10. Zherbun AB, Polianskaya NYu, Nosov FS et al. Host’s Antigens in Purifies Preparations for Flu /Etiology and Specific Prophylaxis of Flu.- Leningrad, 1982.- P.70-81 [Rus]
11. Polianskaya NYu, Sherbun AB. Allontois Neoalbumin Components of Complete Virions Flu Vaccines. Chromatography in Biology and Medicine: Scientific Conference: Abstarcts.- Moscow, P.249-50 (1983) [Rus]
12. Webssters RQ, Zaver WQ. Antigenic Variations of Influenza Viruses// The Influenza Viruses and Influenza (ED Kilbourneed). Academic Res.,209-314 (1983)
13. Zorin NA, Zorina VN. The Role of Proteins from the Family of Macroglobins in Mechanisms of Infection. J Microbiol, Epidemiol Imunol., 3, 105-12 (2003) [Rus]
14. Divocha VO. Cellular Components associated with virus of flu. Odessa Med.J. 2(46), 8-10 (1998) [Ukr]
15. Divocha VO. Changes in Chicken Embryo at the Action of Flu Virus. Odessa Med. J., 2, 100-5 (2000) [Ukr]
16. Divocha VO, Sova YuG., Mikhalchuk VM. Separation and Purification of White Mice Lung Trypsin-like Proteinases. Medical Chem., 3(3), 73-7 (2003) [Ukr]
17. Divocha VA, Sova YuG., Mikelashvili MT. Protective Role of Antiprotease Immune Sera at Experimental Flu. I.I. Mechnikov’s Ideas and the Development of Modern Natural Sciences: Abstracts of Scientific Conference, 28-30, November, Kharkov, 102-3 (1995) [Rus]
18. Divocha VO, Sova YuG, Mihkalchuk BM. Studing of Physical-and-Chemical Properties of Isoenzymes of Trypsin-like Proteinases. Med. Chem.,3(4), 31-4 (2001) [Ukr]
19. Divocha VO. The Method of Inhibitor of Trypsine-like Proteases Obtaining: Patent N 23548 A (Ukraine), IPC6 A61K25/00, 02.06.1998 (Ukr)
20. Divocha VO, Mikelashvili MT, Mikhalcuk VM. The Action of Inhibitor of Trypsin-like Protease on Flu Infection at the Conditions of Experiment. Infect Dis.,- 2,35-9 (2001) [(Ukr]
21. Divocha VO. Inhibitor of Ttrypsin-like Proteases as Antiviral Remedy: Patent N 37324 A (Ukraine), IPC6 F61R31/14, 15.05.2001 (Ukr)
22. Bin Goton, Tomohiko Ogasawara, Tetsuya Tojoda et al. An Endoprotease Homologons to the lood Clotting Factor X as a Determinant of Viral tropism in Chick Emryo, J.Embo, 9,12, 4189-95 (1990)
23. Mikhalcuk VN, Divocha VP, Gozhenko AI. The presence of Trypsin-like Protease and Its Inhibitors in the Outcomes of gamma-Globin manufacturing. Med. Chem, 8, 1,60-3 (2006) [Ukr]
24.Markova OA, Kalashnikova VV, Khvatova VB. Immunoenzyme Method of Antithrombin-3 Detection. Problems Med Chem, 5, 127-30 (1989) [Rus]Podiarene SM, Letskene MN,
25. Podiarene SM, Letskene MN, Mauritsaye MM et al. Immunoenzyme Purification α1-inhibitor of Proteases from Humans Blood Plasma. Probl Virol, 5, 96-9 (1989) [Rus].
26. Cao Tin M., Sung Michael T. A protamine — line domain in basic adenovirus core protein. Biochem. & Biophys. Res. Commun. 108, 3, 1061-1066 (1988)
27. Scheid A., Choppin P. W. Activation of cell fusion and infectivity by proteolytic cleavage of a Sendai virus glycoprotein In “Proteasis and Biological Control” (E. Reich, R. D. Ritkin and E. Shaw, eds.). Cold Spring Harbor. 4, 645-659 (1975)
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/