Journal of Medical Discovery (2017); 2(1): jmd17001; doi:10.24262/jmd.2.1.17001; Received January 8th, 2017, Accepted February 1th, 2017, Published February 20th, 2017.
Hemodynamics and Wall Content in Cerebral Aneurysm: A Mini-Review
Xinjie Duan1,*
1 Mechanical Engineering and Material Science, University of Pittsburgh, PA USA.
* Correspondence: Xinjie Duan, 636 Benedum Hall, 3700 o’hara street, Pittsburgh, PA 15261 Email: xid14@pitt.edu.
Abstract
Cerebral aneurysms are pathological enlargements of the walls of cerebral arteries. Rupture of aneurysms causes 80% of subarachnoid hemorrhages. It is generally accepted that the abnormal hemodynamics within the aneurysm sac can lead to a breakdown in the normal process of collagen renewal and remodeling leaving the aneurysm vulnerable to rupture. However, the link between hemodynamics and wall integrity, as well as the underlying mechanisms governing the aneurysm pathophysiology remain poorly understood. The goal of this paper is to review the current state of knowledge about hemodynamics and wall content of cerebral aneurysms.
Keywords: Cerebral aneurysms, hemodynamics, wall content
Introduction
Cerebral aneurysms are focal dilatations of the wall of cerebral arteries, existing in 2% to 8% of adult population [1]-[4]. 80% to 85% of cerebral aneurysms are located in the anterior circulation, most frequently found at the junction of the internal carotid artery and posterior communicating artery, the anterior communicating-artery complex, and the trifurcation of middle cerebral artery, In the posterior circulation, the basilar artery bifurcation is the most common location of aneurysm [5]. Although the rupture rate of incidental aneurysms is very low (estimated at 0.3-3% per year), rupture of aneurysms causes 80% of subarachnoid hemorrhages (SAH), which is a major clinical problem in the United States, with occurrence of approximately 1 in 10000 people each year. The mortality rate of SAH from ruptured aneurysm is ~50%, including 12% pre-hospital deaths, and one-third of survivors suffer morbidity [6]-[9].
Clinical management
The management of unruptured aneurysms is controversial, because the risk associated with treatment options, including surgical clipping and endovascular therapies, can exceed the natural risk of rupture [10]-[12]. Therefore, treatment or observation is the important decision clinicians and patients often have to make. Aneurysm size, location, aspect ratio, and presence of bleb or blister morphology, as well as patient’s age, family history and smoking status are used in assessing the development, enlargement and rupture of intracranial aneurysms [13]-[18]. Although aneurysm size is widely used as a measure for evaluating rupture risk, a significant number of aneurysms smaller than the critical size (7 mm) were ruptured in previous studies, indicating size is insufficient as a single parameter to assess rupture risk [13], [19]. Improving the clinical management of aneurysm requires an understanding of the underlying mechanisms governing the development, enlargement and rupture of cerebral aneurysms.
Natural history
It is commonly accepted that the natural history of cerebral aneurysms is driven by flow-induced progressive degradation of the wall [20]-[22]. Histological analysis of resected human aneurysm tissue suggested that abnormal aneurysmal flow conditions likely cause endothelial dysfunction, which induces accumulation of cytotoxic and pro-inflammatory substances in the wall as well as thrombus formation, that leads to loss of mural cells and wall degeneration [21]. However, the mechanisms and interactions between the various factors involved in the evolution of cerebral aneurysms remain poorly understood [20].
Rupture risk assessment
A large body of research has been focused on identifying a link between rupture and hemodynamic parameters such as magnitude of wall shear stress (WSS) and oscillatory shear index (OSI) [23]-[29]. However, to date these results are inconsistent and have not identified a clinically useful hemodynamic marker. An underlying assumption of this past work is the cells necessary to sense and respond to hemodynamic loads exist within the aneurysm sac. However, this has been shown not to be the case. In a large percentage of aneurysms, the endothelium is missing [21]. In many of these aneurysms, the wall is hypo-cellular and hence has lost its capacity for wall maintenance and remodeling. Clearly, there are different stages of aneurysm pathology, with some stages lacking the means to sense and respond to hemodynamic loading [30]. The interconnection between intra-aneurysmal hemodynamics and wall structure has never been studied. Until we have a more integrated understanding of hemodynamics, wall structure and wall strength, it is unlikely we will be able use patient specific hemodynamics to gauge rupture risk.
Wall structure
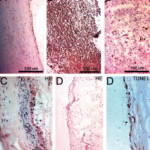
There are at least three categories of unruptured aneurysm wall with great variability in intramural structure including cellular content and organization: (A) endothelialized wall with linearly organized SMC, (B) thickened wall with disorganized smooth muscle cells (SMC), and (C) hypocellular wall with either myointimal hyperplasia or organizing luminal thrombosis, as shown in Figure 1 A, B, and C. The extracellular matrix is manufactured by SMCs and fibroblast cells, so we would expect the heterogeneity in collagen architecture and therefore mechanical properties within the unruptured aneurysms due to this variability in mural content.
Figure 1. Four aneurysm wall types identified. A, Endothelialized wall with linearly organized SMCs; B, Thickened wall with disorganized SMC; C, Hypocellular wall with either intimal hyperplasia or organizing luminal thrombosis; D. An extremely thin thrombosis-lined hypocellular wall. Only type D is 100% associated with ruptured aneurysms. (Reproduced with permission from [21], Copyright Wolters Kluwer Health, Inc.)
Hemodynamics
A large variety of flow patterns in unruptured aneurysms was found [25]-[27]. Four flow types with very difference inflow and vortex characteristics were reported by Cebral et al. in unruptured aneurysms, unchanging direction of inflow jet with one vortex; unchanging direction of inflow jet with multiple associated vortices lasting during the whole cardiac cycle; changing direction of inflow jet with one vortex; changing direction of the inflow jet with creation or destruction of multiple vortices [27]. More recently, Cebral’s group has developed a quantitative method for scoring IA flows [31]. A comprehensive effort that combines histological analysis and computational fluid dynamics are needed to understand how hemodynamics are tied to wall structure in human cerebral aneurysms [32-34].
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
None
References
1. Brisman, J. L., Song, J. K., & Newell, D. W. (2006). Cerebral aneurysms. New England Journal of Medicine, 355(9), 928-939.
2. Stehbens, W. E. Pathology of the Cerebral Blood Vessels. St. Louis: C.V. Mosby Co., 1972.
3. Rinkel, G., M. Djibuti, A. Agra, and J. V. Gijn. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29:251–256, 1998.
4. Wiebers, D. O., J. C. Torner, and I. Meissner. Impact of unruptured intracranial aneurysms on public health in the United States. Stroke 23:1416–1419, 1992.
5. Schievink, W. I. (1997). Intracranial aneurysms. New England Journal of Medicine, 336(1), 28-40.
6. Kelly, P. J., Stein, J., Shafqat, S., Eskey, C., Doherty, D., Chang, Y., … & Furie, K. L. (2001). Functional recovery after rehabilitation for cerebellar stroke. Stroke, 32(2), 530-534.
7. Ropper, A. H., & Zervas, N. T. (1984). Outcome 1 year after SAH from cerebral aneurysm: management morbidity, mortality, and functional status in 112 consecutive good-risk patients. Journal of neurosurgery, 60(5), 909-915.
8. Van Gijn, J., & Rinkel, G. J. E. (2001). Subarachnoid haemorrhage: diagnosis, causes and management. Brain, 124(2), 249-278.
9. Inghall, T.J. and Whisnant J.P.. Subarachnoid hemorrhage. In Epidemiology of Subarachnoid Haemorrhage, pages 63–78. Marcel-Dekker, 1998.
10. The International Study of Unruptured Intracranial Aneurysms Investigators (1998) Unruptured Intracranial Aneurysms–Risk of Rupture and Risks of Surgical Intervention. N Engl J Med 339(24):1725–33.
11. Broderick, J. P., Brown, R. D., Sauerbeck, L., Hornung, R., Huston, J., Woo, D., … & Connolly, E. S. (2009). Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke 40(6):1952–1957
12. Wiebers, D. O., & International Study of Unruptured Intracranial Aneurysms Investigators. (2003). Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. The Lancet,362(9378), 103-110.
13. Lall, R. R., Eddleman, C. S., Bendok, B. R., & Batjer, H. H. (2009). Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus 26(5):E2
14. Ujiie, H., Tamano, Y., Sasaki, K., & Hori, T. (2001). Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm?. Neurosurgery, 48(3), 495-503.
15. Xiang, J., Natarajan, S. K., Tremmel, M., Ma, D., Mocco, J., Hopkins, L. N., … & Meng, H. (2011). Hemodynamic–morphologic discriminants for intracranial aneurysm rupture. Stroke, 42(1), 144-152.
16. Ishibashi, T., Murayama, Y., Urashima, M., Saguchi, T., Ebara, M., Arakawa, H., … & Abe, T. (2009). Unruptured intracranial aneurysms incidence of rupture and risk factors. Stroke, 40(1), 313-316.
17. Juvela, S., Porras, M., & Poussa, K. (2000). Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture.Journal of neurosurgery, 93(3), 379-387.
18. Utter, B., & Rossmann, J. S. (2007). Numerical simulation of saccular aneurysm hemodynamics: influence of morphology on rupture risk. Journal of biomechanics, 40(12), 2716-2722.
19. Weir, B., Macdonald, R. (1996). Neurosurgery, McGraw-Hill, Intracranial aneurysms and hemorrhage: An overview, 2191–2213.
20. Cebral, J. R., & Raschi, M. (2013). Suggested connections between risk factors of intracranial aneurysms: A review. Ann Biomed Eng 41(7):1366–1383.
21. Frösen, J., Piippo, A., Paetau, A., Kangasniemi, M., Niemelä, M., Hernesniemi, J., Jääskeläinen, J. (2004). Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke 35(10):2287–93
22. Humphrey, J. D., Canham, P. B. (2000). Structure, mechanical properties, and mechanics of intracranial saccular aneurysms. J Elasticity 61(1-3):49-81.
23. Cebral, J.R., et al., Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR. American journal of neuroradiology. 2011. 32(2): p. 264-70.
24. Cebral, J. R., Mut, F., Sforza, D., Löhner, R., Scrivano, E., Lylyk, P., & Putman, C. (2011). Clinical Application of Image-Based CFD for Cerebral Aneurysms. International Journal for Numerical Methods in Biomedical Engineering, 2011. 27(7): p. 977-992.
25. Boussel, L., Rayz, V., Martin, A., Acevedo, Bolton, G., Lawton, M. T., Higashida, R., … & Saloner, D. (2009). Phase contrast magnetic resonance imaging measurements in intracranial aneurysms in vivo of flow patterns, velocity fields, and wall shear stress: Comparison with computational fluid dynamics. Magnetic Resonance in Medicine, 61(2), 409-417.
26. Castro, M. A., Putman, C. M., Sheridan, M. J., & Cebral, J. R. (2009). Hemodynamic patterns of anterior communicating artery aneurysms: a possible association with rupture. American journal of neuroradiology, 30(2), 297-302.
27. Cebral, J. R., Castro, M. A., Burgess, J. E., Pergolizzi, R. S., Sheridan, M. J., and Putman, C. M., 2005, Characterization of Cerebral Aneurysms for Assessing Risk of Rupture By Using Patient-Specific Computational Hemodynamics Models. AJNR. Am J Neuroradiology, 26(10), pp. 2550-2559.
28. Steinman, D. A., Milner, J. S., Norley, C. J., Lownie, S. P., & Holdsworth, D. W. (2003). Image-based computational simulation of flow dynamics in a giant intracranial aneurysm. American Journal of Neuroradiology, 24(4), 559-566.
29. Jou, L. D., Lee, D. H., Morsi, H., & Mawad, M. E. (2008). Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. American Journal of Neuroradiology, 29(9), 1761-1767.
30. Robertson, A. M., Watton, P. N. (2012). Computational fluid dynamics in aneurysm research: critical reflections, future directions. AJNR Am J Neuroradiol 33(6):992–995
31. Byrne, G., Mut, F., & Cebral, J. (2014). Quantifying the large-scale hemodynamics of intracranial aneurysms. American Journal of Neuroradiology, 35(2), 333-338.
32. Robertson, A. M., Duan, X., Aziz, K. M., Hill, M. R., Watkins, S. C., & Cebral, J. R. (2015). Diversity in the strength and structure of unruptured cerebral aneurysms. Annals of biomedical engineering, 43(7), 1502-1515.
33. Cebral, J. R., Duan, X., Chung, B. J., Putman, C., Aziz, K., & Robertson, A. M. (2015). Wall mechanical properties and hemodynamics of unruptured intracranial aneurysms. American Journal of Neuroradiology, 36(9), 1695-1703.
34. Cebral, J. R., Duan, X., Gade, P. S., Chung, B. J., Mut, F., Aziz, K., & Robertson, A. M. (2016). Regional mapping of flow and wall characteristics of intracranial aneurysms. Annals of biomedical engineering, 44(12), 3553-3567.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/