Journal of Medical Discovery (2016); 1(1): jmd16010; doi:10.24262/jmd.1.1.16010; Received November 9th, Revised November 28th, Accepted November 28th, Published November 29th.
Differential expression of miR-21, miR-133 and miR-155 from exosome fractions isolated from oral squamous cell carcinomas in vitro
Brady Petersen1, Karl Kingsley2*
1Department of Clinical Sciences at the University of Nevada, Las Vegas – School of Dental Medicine, 1700 W Charleston Avenue, Las Vegas, Nevada, 89106, USA, (702) 774-2466; 2Department of Biomedical Sciences at the University of Nevada, Las Vegas – School of Dental Medicine, 1001 Shadow Lane, Las Vegas, Nevada, 89106, USA.
* Correspondence: Karl Kingsley, University of Nevada, Las Vegas–School of Dental Medicine, 1001 Shadow Lane B227, Las Vegas, Nevada 89106, USA, Tel: (702) 774-2623; Fax:(702) 774-2721; Email:Karl.Kingsley@unlv.edu
Abstract
Exosomes derived from oral cancer cells are membranous vesicles secreted into the surrounding extracellular environment, which are now known to regulate and modulate oral squamous cell carcinoma (OSCC) progression through the horizontal transfer of bioactive molecules, including proteins, lipids and microRNA (miRNA). To date, only one study has demonstrated the secretion of exosomes from cultured OSCC cells, which could potentially facilitate research and possible new treatment modalities. Based upon this information, the primary goal of this study was to examine the potential to isolate and evaluate exosomes from oral cancer cell lines, as well as normal non-cancerous controls.
The OSCC cell lines SCC4, SCC9, SCC15, SCC25, CAL27 and the normal gingival cell line HGF-1 were cultured for supernatant collection, which was subsequently centrifuged to remove all intact, but non-adherent cells. RNA was then extracted from the supernatant, as well as from the cytoplasm from each cell line. Molecular screening using primers specific for miRNA to miR-16, -21, -122, -133 and -155 revealed differential expression of miR-21, miR-133 and miR-155 in the cellular fraction of the OSCC cell line, with differential expression of miR-16 in HGF-1 cells. Analysis of supernatant fractions required repeated concentration via centrifugation to detect exosome miRNA, including miR-21, miR-133 and miR-155 from SCC25 supernatant but only miR-16 was detected in the supernatant from HGF-1 cells. Because most cases of OSCC are detected in advanced stages, finding a reliable, non-invasive early stage diagnostic marker would facilitate screening and increase possible treatments.
Keywords: exosomes, miR-21, miR-133, miR-155, microRNA, HGF, oral cancer
Introduction
Oral cancer is the sixth most common cancer in the United States, with oral squamous cell carcinoma (OSCC) in ~90% of oral cancer patients [1,2]. If OSCC is detected at the early stages, the 5-year survival rate is close to 80% [3,4]. However, if OSCC is detected at the later stages, the 5-year survival rate decreases to 20–40% [5,6]. This supports the necessity for early detection methods for increasing long-term patient survival. The detection of microRNAs (miRNAs) in human saliva has recently become an emerging field for monitoring oral diseases using salivary diagnostics [7,8]. Exosomes are 40-120 nm membranous vesicles that contain unique subsets of miRNA [9]. Most cell types secrete exosomes and they can be found in abundance in body fluids such as blood, urine, and saliva [10]. Exosomes derived from oral cancer cells, also called Oncosomes, secreted into the surrounding extracellular environment, are now known to regulate and modulate OSCC progression through the horizontal transfer of bioactive molecules, including proteins, lipids and miRNA [11,12]. Studies that are able to demonstrate the secretion of exosomes from cultured OSCC cells could potentially facilitate research and possible new treatment modalities.
Studies have recently demonstrated that discriminatory salivary biomarkers can be readily detected upon the development of systemic diseases like pancreatic cancer, breast cancer, lung cancer, and ovarian cancer [13-16]. The challenge is learning more about how discriminatory biomarkers for diseases developing distally from the oral cavity would appear in saliva [17]. Exosomes are likely biomarker candidates as more research is finding out that functional miRNA can be isolated from saliva samples [18]. Very little is known, however, about which miRNAs are up-regulated or down-regulated in oral cancer and whether or not salivary biomarkers can be used as an oral cancer diagnostic tool [19].
Based upon this information, the primary goal of this study was to examine the potential to isolate and evaluate exosomes from oral cancer cell lines, as well as normal non-cancerous control and explore the potential for salivary exosomes to be used in the future as a reliable, non-invasive early stage diagnostic marker that would facilitate screening and increase possible OSCC treatments.
Materials and Methods
Cell culture and cell lines
The human oral squamous cell carcinoma cell lines SCC4 (CRL-1624), SCC9 (CRL-1629), SCC15 (CRL-1623), CAL 27 (CRL-2095) and SCC25 (CRL-1628), were obtained from American Type Culture Collection (ATCC: Manassas, VA). CAL 27 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) with 4.0 mM L-Glutamine adjusted to contain 3.7 g/L sodium bicarbonate, 4.5 g/L glucose, and 110 mg/L sodium pyruvate, obtained from HyClone (Logan, UT). SCCC4, SCC9, SCCC15 and SCC25 cells were maintained in a 1:1 mixture of DMEM and Ham’s F12 medium with 2.5 mM L-Glutamine, modified to contain 15 mM 4-2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), 0.5 mM sodium pyruvate, and 1.2 g/L sodium bicarbonate.
The normal oral gingival fibroblast cell line, HGF-1 (CRL-2014), was also obtained from American Type Culture Collection (ATCC: Manassas, VA). HGF-1 cells were maintained in DMEM with 4 mM L-Glutamine, adjusted to contain 3.7 g/L sodium bicarbonate and 4.5 g/L glucose, from HyClone (Logan, UT). All cell culture media was supplemented with 1% Penicillin (10,000 units/mL)-Streptomycin (10,000 g/mL) solution and 10% fetal bovine serum (FBS) obtained from HyClone (Logan, UT). Cells were cultured in 75 cm2 BD Falcon tissue-culture treated flasks (Bedford, MA) at 37°C and 5% CO2 in humidified chambers.
RNA isolation
To evaluate microRNA, cellular and supernatant (exosome-containing) fractions were isolated using centrifugation 2.1 RCF or g (4,800 RPM × 10 minutes). RNA was isolated from 1.5 × 107 cells from all cell lines (SCC4, SCC9, SCC15, SCC25, CAL27 and HGF-1) after 72 hours of cell growth using ABgene Total RNA Isolation Reagent (Epsom, Surrey,UK) and the procedure recommended by the manufacturer. RNA concentration and purity were calculated using UV spectroscopy. The absorbance of diluted RNA samples (10 μL of RNA sample in 490 μL nuclease-free water, pH 7.0) was measured at 260 and 280 nm. RNA purity was determined by calculating the ratio of A260:A280, which should be > 1.80. Concentration for RNA samples was determined by the A260 reading of 1 = 40 μg/mL RNA, based on an extinction coefficient calculated for RNA in nuclease-free water. Concentration was calculated as 40 × A260 absorbance measure x dilution factor (50). Total yield was determined by concentration × sample volume in mL. (Example: RNA standard A260 = 0.75; Concentration = 40 × 0.75 × 50 = 1,500 μg/mL; Yield = 1,500 μg/mL * 1.0 mL = 1,500 μg or 1.5 mg RNA). Similar RNA concentrations were obtained from each cell line, which ranged from 876 – 955 ng/L. Analysis of A260/A280 ratio, confirmed the purity, which ranged between 1.66 and 1.88.
The exosome-containing supernatant fractions were prepared as described above (centrifugation at 2,100 g (RCF) × 10 minutes and were then serially centrifuged at 4,800 g (RCF) * 10 minutes and then 10,000 g (RCF) × 15 minutes to ensure no cells or cellular fractions were present. The identical reagent amounts and procedures were then used to isolate RNA from the processed exosome-containing supernatant fractions, although this did not include counting cells since these had been removed through centrifugation prior to this procedure. RNA concentrations obtained from the supernatant fractions were significantly lower, ranging between 88 – 191 ng/L. Purity ranged between 1.47 – 1.72, which was within the acceptable range for the one-step RT-PCR kit utilized (recommended range: 1.5 – 2.0)
Quantitative Real time PCR (RT-PCR)
Reverse transcription and polymerase chain reaction (RT-PCR) was performed with the ABgene Reverse-iT One-Step RT-PCR Kit (ReadyMix Version) and a Mastercycler gradient thermocycler (Eppendorf: Hamburg, Germany) on both the cellular and exosome-containing supernatant fractions. The primers for the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [20] were used to confirm cellular RNA isolation as well as to confirm the absence of cells from the exosome-containing supernatant fractions, which were synthesized by SeqWright (Houston, TX) (Table 1).
In brief, one μg of template (total) RNA was used for each reaction from the cellular RNA extractions derived from each cell line. The reverse transcription step ran for 30 minutes at 47°C, followed by denaturation for 2 minutes at 94°C. Thirty five amplification cycles were run, consisting of 20 second denaturation at 94°C, 30 seconds of annealing at 58.2°C, and 6.5 minutes of extension at 72°C. Final extension was run for 5 minutes at 72°C. Reaction products were separated by gel electrophoresis using Reliant 4% NuSieve R3:1 Plus Agarose gels (Lonza: Rockland, ME). Bands were visualized by UV illumination of ethidium-bromide-stained gels and captured using a Kodak Gel Logic 100 Imaging System and 1D Image Analysis Software (Eastman Kodak: Rochester, NY). Quantifictation of RT-PCR band densitometry was performed using Adobe (San Jose, CA) Photoshop imaging software, Image Analysis tools.
The sequences of primers used for the experimental screening for microRNA specific to miR-16 [21], miR-21 [22], miR-133 [23], and miR-155 [24] were listed in table 1.
| Name | Sequence | Tm(℃) | Optimal Tm(℃) |
| GAPDH-Forward | ATCTTCCAGGAGCGAGATCC | 58.2 | 53.2 |
| GAPDH-Reverse | ACCACTGACACGTTGGCAGT | 60.6 | |
| miR-16-Forward | TAGCAGCACGTAAATATTGGCG | 60.8 | 54.3 |
| miR-16-Reverse | TGCGTGTCGTGGAGTC | 59.3 | |
| miR-21-Forward | GCCACCACACCAGCTAATTT | 60.4 | 55.5 |
| miR-21-Reverse | CTGAAGTCGCCATGCAGATA | 60.4 | |
| miR-133-Forward | ccggttaactcgagctctgtgagaggttagtcag | 71.9 | 65.7 |
| miR-133-Reverse | ctagctaggaattctgtgacctgtgaacttgggc | 70.7 | |
| miR-155-Forward | ttaatgctaattgtgataggggt | 57.4 | 50.6 |
| miR-155-Reverse | cctatcacaattagcattaatt | 55.6 |
Table 1. Sequences of quantitative Real time PCR primers
Results
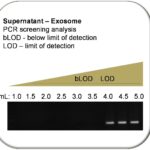
These results demonstrated that RNA concentrations from the cellular fractions were significantly higher, ranging from 876 – 955 ng/uL (Ave.=918.2 ng/uL), compared with those from the exosome-containing supernatant fractions, which ranged between 88 – 191 ng/uL (Ave.= 129.1 ng/uL). These data suggested that standard RT-PCR may not be sensitive enough to detect RNA at concentrations approximately seven-fold lower than recommended (1 ug/uL). To test this hypothesis, PCR signals were evaluated after serially combining fractions each derived from 1 mL aliquots of centrifuged supernatant (Figure 1). These results demonstrated that RT-PCR performed on RNA isolated from 1.0 to 3.5 mL of supernatant remained below the limit of detection (bLOD), but serial combination of 4.0 – 5.0 mL of centrifuged supernatant was sufficient to produce an RT-PCR signal from the normal HGF-1 cell line using miR-16 primers.
Figure 1. Screening of HGF-1 (normal cell) supernatant for miR-16. RNA concentrations from the non-cellular, exosome-containing supernatant fractions averaging 129.1 (range 88 – 191 ng/uL) were significantly lower than cellular RNA concentrations, averaging 918.2 ng/uL (range 876-955). The low levels of RNA in the non-cellular, exosome-containing supernatant fraction (below the limit of detection, bLOD) required the serial combination of multiple supernatant 1mL fractions for sufficient concentrations of microRNA to enable PCR detection of miR-16 from normal gingiva HGF-1 cells.
Once these parameters were established, molecular screening using primers specific for miRNA to miR-16, miR-21, miR-133 and miR-155 were performed on all cell lines, including the oral squamous cell carcinoma cell lines SCC4, SCC9, SCC15, SCC25, CAL27 and the normal gingival cell line HGF-1 (Figure 2). These results demonstrate expression and extracellular export of miR-16 from the supernatant of HGF-1 only. Differential expression of miR-21, miR-133 and miR-155 was observed in both the cellular and supernatant fractions of the oral cancer cell lines and was not observed in the HGF-1 (normal cell) control. More specifically, miR-21 was expressed and exported into the supernatant from most of the oral cancer cell lines, including SCC4, SCC9, SCC25 and CAL27 – although it was not observed with SCC15. Positive results using miR-133 were also observed from the supernatant derived from SCC4, SCC9, SCC25 and SCC15 – although it was not observed with CAL27. Finally, PCR using miR-155 revealed expression and secretion among SCC4, SCC9 and SCC25 cells – but not in either CAL27 or SCC15.
Figure 2. Screening of supernatant from all oral cancer cell lines for microRNA. Using the established parameters (LOD>4mL) centrifuged and serially concentrated RNA from supernatant fractions was screened. Using RT-PCR for presence of expressed and exported miRNA-16,-21,-133, and -155. miR-16 was detected only from supernatant derived from HGF-1 (normal) cells, while differential results were obtained from SCC4, SCC9, SCC15, SCC25 and CAL27 cells. Positive expression of miR-21 and miR-133 was found among most oral cancer cells (4/5) with more limited miR-155 expression (3/5).
Discussion
Based upon the paucity of evidence regarding which miRNAs are up-regulated or down-regulated in oral cancer and whether or not salivary biomarkers can be used as an oral cancer diagnostic tool, the primary objective of this study was to examine the potential to isolate and evaluate exosomes from oral cancer cell lines, as well as normal non-cancerous cell controls. This information could be useful to determine if salivary exosomes might be used in the future as a reliable, non-invasive early stage diagnostic marker that would facilitate screening and increase early diagnosis and thereby allow more effective treatments. The results of this study indicated that exosomes and microRNA from oral cancer cells in culture can, in fact, be isolated and screened using ordinary laboratory equipment and procedures. Furthermore, these results correspond with new evidence and pilot studies that have suggested saliva-derived miRNA and exosomes may be useful as biomarkers for oral cancers [25,26].
This study also revealed that microRNA expression in oral tumors may be variable and differential, even among commercially available oral cancer cell lines, which suggest that further research is needed to determine which miRNA correspond with potential clinical diagnostics and therapeutic treatments [27,28]. For example, the results of this study appear to confirm the expression of miR-21, miR-133 and miR-155 in most of the oral cancers screened – a result that corresponds with other recent studies of oral cancer [22,29-31]. However, additional studies are now revealing ever more complex and nuanced miRNA expression patterns that may reveal other potential biomarkers for oral cancer, including miR-139, miR-223, and miR-375 [32-36].
Conclusion
This study showed that miRNA from exosomes can be isolated and detected from cultured oral cancer supernatant; however, this process requires repeated concentration to enable detection. The detection of miR-16 only in normal non-cancerous cell supernatant (exosomes) appears to confirm a previous report of five consistent, abundantly expressed microRNAs from human whole saliva (miR-16, miR-24, miR-191, miR-203, miR-223) taken from healthy patients [37]. Although these data provide some evidence for the use of miR-21, -133, and -155 as oral cancer biomarkers, these findings raise more questions as to why these were differentially expressed in oral squamous cell carcinoma supernatant (exosomes). This may suggest more information is needed to understand why these particular miRNAs are differentially regulated in oral cancer cells lines. These data contribute important data towards the elucidation of reliable, non-invasive early stage diagnostic markers that may facilitate screening non-invasive saliva-based screening. This study supports the initial finding that tumor-derived exosomes can be analyzed from in vitro cell cultures, which may allow for further development of discriminatory biomarkers from other pre-malignant and malignant cell cultures that can be applied to saliva and other fluid diagnostic platforms.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
BP and KK would like to thank the UNLV Graduate and Professional Student Association and the UNLV-SDM Office of Research for their support with this project.
References
- Saman DM. Head Neck Oncol. A review of the epidemiology of oral and pharyngeal carcinoma: update.2012 Jan 13;4:1. doi: 10.1186/1758-3284-4-1. Review. PMID: 22244087
- Vigneswaran N, Williams MD. Oral Maxillofac Surg Clin North Am. Epidemiologic trends in head and neck cancer and aids in diagnosis.2014 May;26(2):123-41. doi: 10.1016/j.coms.2014.01.001. Review. PMID: 24794262
- Kumar M, Nanavati R, Modi TG, Dobariya C. J Cancer Res Ther. Oral cancer: Etiology and risk factors: A review.2016 Apr-Jun;12(2):458-63. doi: 10.4103/0973-1482.186696. Review. PMID: 27461593
- Amit M, Yen TC, Liao CT, et al.; International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Cancer. Improvement in survival of patients with oral cavity squamous cell carcinoma: An international collaborative study.2013 Dec 15;119(24):4242-8. doi: 10.1002/cncr.28357. PMID: 24114787
- Chen WC, Lai CH, Fang CC, Yang YH, Chen PC, Lee CP, Chen MF. Medicine (Baltimore). Identification of High-Risk Subgroups of Patients With Oral Cavity Cancer in Need of Postoperative Adjuvant Radiotherapy or Chemo-Radiotherapy.2016 May;95(22):e3770. doi: 10.1097/MD.0000000000003770. PMID: 27258508
- Ravindran G, Sawant SS, Hague A, Kingsley K, Devaraj H. Head Neck. Association of differential β-catenin expression with Oct-4 and Nanog in oral squamous cell carcinoma and their correlation with clinicopathological factors and prognosis.2015 Jul;37(7):982-93. doi: 10.1002/hed.23699. PMID: 24700702
- Ding Y, Ma Q, Liu F, Zhao L, Wei W. PLoS One. The Potential Use of Salivary miRNAs as Promising Biomarkers for Detection of Cancer: A Meta-Analysis.2016 Nov 10;11(11):e0166303. doi: 10.1371/journal.pone.0166303. PMID: 27832115
- Yoshizawa JM, Wong D. Salivary MicroRNAs and Oral Cancer Detection. Methods Mol Biol. 2013 ; 936: 313–324.
- Vyas N, Dhawan J. Cell Mol Life Sci. Exosomes: mobile platforms for targeted and synergistic signaling across cell boundaries.2016 Nov 8. [Epub ahead of print] Review. PMID: 27826642
- Desdín-Micó G, Mittelbrunn M. Cell Adh Migr. Role of exosomes in the protection of cellular homeostasis.2016 Nov 22:1-8. [Epub ahead of print] PMID: 27875097
- Yakob M, Fuentes L, Wang MB, Abemayor E, Wong DT. Curr Oral Health Rep. Salivary biomarkers for detection of oral squamous cell carcinoma – current state and recent advances.2014 Jun 1;1(2):133-141. PMID: 24883261
- Principe S, Hui AB, Bruce J, Sinha A, Liu FF, Kislinger T. Proteomics. Tumor-derived exosomes and microvesicles in head and neck cancer: implications for tumor biology and biomarker discovery.2013 May;13(10-11):1608-23. doi: 10.1002/pmic.201200533. Review. PMID: 23505015
- Takahashi RU, Miyazaki H, Ochiya T. Cancers (Basel). The Roles of MicroRNAs in Breast Cancer.2015 Apr 9;7(2):598-616. doi: 10.3390/cancers7020598. Review. PMID: 25860815
- Rolfo C, Castiglia M, Hong D, et al. Biochim Biophys Acta. Liquid biopsies in lung cancer: the new ambrosia of researchers.2014 Dec;1846(2):539-46. doi: 10.1016/j.bbcan.2014.10.001. PMID: 25444714
- Lau C, Kim Y, Chia D, Spielmann N, Eibl G, Elashoff D, et al. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J Biol Chem. 2013; 288(37):26888-97.
- Dorayappan KD, Wallbillich JJ, Cohn DE, Selvendiran K. Gynecol Oncol. The biological significance and clinical applications of exosomes in ovarian cancer.2016 Jul;142(1):199-205. doi:10.1016/j.ygyno.2016.03.036. Review. PMID: 27058839
- Schwarzenbach H. Expert Rev Mol Diagn. The clinical relevance of circulating, exosomal miRNAs as biomarkers for cancer.2015;15(9):1159-69. doi: 10.1586/14737159.2015.1069183. Review. PMID: 26202667
- Silva A, Bullock M, Calin G. Cancers (Basel). The Clinical Relevance of Long Non-Coding RNAs in Cancer.2015 Oct 27;7(4):2169-82. doi: 10.3390/cancers7040884. Review. PMID: 26516918
- Lin N, Lin Y, Fu X, et al. Clin Lab. MicroRNAs as a Novel Class of Diagnostic Biomarkers in Detection of Oral Carcinoma: a Meta-Analysis Study.2016;62(3):451-61. PMID: 27156336
- Moody M, Le O, Rickert M, et al. Cancer Cell Int. Folic acid supplementation increases survival and modulates high risk HPV-induced phenotypes in oral squamous cell carcinoma cells and correlates with p53 mRNA transcriptional down-regulation.2012 Mar 23;12:10. doi: 10.1186/1475-2867-12-10. PMID: 22443202
- Xia L, Zhang D, Du R, et al. Int J Cancer. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. 2008 Jul 15; 123(2):372-9. PMID: 18449891
- Hedbäck N, Jensen DH, Specht L, et al. PLoS One. MiR-21 expression in the tumor stroma of oral squamous cell carcinoma: an independent biomarker of disease free survival. 2014 Apr 22; 9(4):e95193. eCollection 2014. PMID: 24755828
- Wu D, Pan H, Zhou Y, Zhou J, Fan Y, Qu P. Mol Med Rep. microRNA-133b downregulation and inhibition of cell proliferation, migration and invasion by targeting matrix metallopeptidase-9 in renal cell carcinoma. 2014 Jun; 9(6):2491-8. Epub 2014 Apr 4. PMID: 24714873
- Shi LJ, Zhang CY, Zhou ZT, et al. Head Neck. MicroRNA-155 in oral squamous cell carcinoma: Overexpression, localization, and prognostic potential. 2014 Apr 2. [Epub ahead of print] PMID: 24692283
- Duz MB, Karatas OF, Guzel E, et al. Cell Oncol (Dordr). Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: a pilot study. 2016 Apr;39(2):187-93. doi: 10.1007/s13402-015-0259-z. PMID: 26650483
- Tian X, Chen Z, Shi S, Wang X, Wang W, Li N, Wang J. Medicine (Baltimore). Clinical Diagnostic Implications of Body Fluid MiRNA in Oral Squamous Cell Carcinoma: A Meta-Analysis.2015 Sep;94(37):e1324. doi: 10.1097/MD.0000000000001324. PMID: 26376377
- Dumache R, Rogobete AF, Andreescu N, Puiu M. Clin Lab. Genetic and Epigenetic Biomarkers of Molecular Alterations in Oral Carcinogenesis. 2015;61(10):1373-81. Review. PMID: 26642697
- Zahran F, Ghalwash D, Shaker O, Al-Johani K, Scully C. Oral Dis. Salivary microRNAs in oral cancer.2015 Sep;21(6):739-47. doi: 10.1111/odi.12340. PMID: 25784212
- Coutinho-Camillo CM, Lourenço SV, de Araújo Lima L, Kowalski LP, Soares FA. Cancer Genet. N29 Expression of apoptosis-regulating miRNAs and target mRNAs in oral squamous cell carcinoma. 2015 Jul-Aug;208(7-8):382-9. doi: 10.1016/j.cancergen.2015.04.004. PMID: 26027785
- Jamali Z, Asl Aminabadi N, Attaran R, Pournagiazar F, Ghertasi Oskouei S, Ahmadpour F. Oral Oncol. MicroRNAs as prognostic molecular signatures in human head and neck squamous cell carcinoma: a systematic review and meta-analysis.2015 Apr;51(4):321-31. doi: 10.1016/j.oraloncology.2015.01.008. Review. PMID: 25677760
- Wu K, Li L, Li S. Tumour Biol. Circulating microRNA-21 as a biomarker for the detection of various carcinomas: an updated meta-analysis based on 36 studies.2015 Mar;36(3):1973-81. doi: 10.1007/s13277-014-2803-2. PMID: 25527152
- Wang L, Jiang H, Li W, et al. Arch Oral Biol. Overexpression of TP53 mutation-associated microRNA-182 promotes tumor cell proliferation and migration in head and neck squamous cell carcinoma.2016 Sep 30;73:105-112. doi: 10.1016/j.archoralbio.2016.09.012. [Epub ahead of print] PMID: 27744260
- Harrandah AM, Fitzpatrick SG, Smith MH, Wang D, Cohen DM, Chan EK. Oral Surg Oral Med Oral Pathol Oral Radiol. MicroRNA-375 as a biomarker for malignant transformation in oral lesions. 2016 Dec;122(6):743-752.e1. doi: 10.1016/j.oooo.2016.07.022. PMID: 27720656
- Tachibana H, Sho R, Takeda Y, et al. PLoS One. Circulating miR-223 in Oral Cancer: Its Potential as a Novel Diagnostic Biomarker and Therapeutic Target.2016 Jul 21;11(7):e0159693. doi: 10.1371/journal.pone.0159693. PMID: 27441818
- He Q, Chen Z, Cabay RJ, et al. Oral Oncol. microRNA-21 and microRNA-375 from oral cytology as biomarkers for oral tongue cancer detection.2016 Jun;57:15-20. doi: 10.1016/j.oraloncology.2016.03.017. PMID: 27208839
- Duz MB, Karatas OF, Guzel E, et al. Cell Oncol (Dordr). Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: a pilot study.2016 Apr;39(2):187-93. doi: 10.1007/s13402-015-0259-z. PMID: 26650483
- Patel RS, Jakymiw A, Yao B, et al. Arch Oral Biol. High resolution of microRNA signatures in human whole saliva.2011 Dec;56(12):1506-13. doi: 10.1016/j.archoralbio.2011.05.015. PMID: 21704302
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/