Journal of Medical Discovery (2016); 1(1): jmd16013; Received November 17th, 2016, Revised December 22nd, 2016, Accepted December 23rd, 2016, Published February 6th, 2017.
Updates on Hepatic Stellate Cell Involvement in Liver Fibrosis
Hui Jing Lim1, Rhun Yian Koh1, Chooi Ling Lim1,*
1 School of Health Sciences, International Medical University, Kuala Lumpur, Malaysia, 57000.
* Correspondence: Chooi Ling Lim. Address: International Medical University, No. 126, Jalan Jalil Perkasa 19, Bukit Jalil, 57000 Kuala Lumpur, Malaysia. Tel/Fax: +60327317611 / +60386561018. E-mail: chooi_linglim@imu.edu.my.
Abstract
Liver fibrosis is known to be an intrinsic healing response to chronic injury characterised by excessive accumulation of extracellular matrix (ECM). Activation of hepatic stellate cells (HSCs) followed by transdifferentiation into myofibroblasts represent the primary events in the development of liver fibrosis as they are the chief ECM-producing cells. Upon liver injury, various mediators are produced by neighbouring cells such as macrophages and damaged hepatocytes, triggering this transdifferentiation. Activated HSCs continue to receive stimuli and perform actions essential for reparation including chemotaxis, proliferation, ECM synthesis and contraction. Reversion of activated HSCs to its quiescent state or clearing by apoptosis only occurs after completion of the reparation process. However, HSCs are activated continuously during chronic liver injury, leading to extensive scar formation which eventually result in liver failure. This review aims to provide updates on the emerging investigations on the role of HSCs in liver fibrosis and to highlight some recent advancements in potential HSC-targeted therapeutic strategies.
Keywords: liver fibrosis, hepatic stellate cells
Introduction
Liver fibrosis is characterised by excessive deposition of extracellular matrix (ECM) as a wound healing response to chronic injury. Discovery of hepatic stellate cell (HSC) activation as the central event has been beneficial in understanding the development of liver fibrosis. In spite of the involvement of other liver cells such as hepatocytes, Kupffer cells, sinusoidal endothelial cells and portal fibroblasts in hepatic fibrogenesis, it has been firmly established that HSC is the primary contributor to all types of liver fibrosis in recent studies [1,2].
HSCs are situated in the subendothelial space of Disse. Under normal physiological conditions, they remain quiescent and play crucial role in homeostasis and storage of lipid-soluble vitamin A. Upon liver injury, HSCs are activated by various injurious stimuli, transdifferentiating into myofibroblasts and subsequently result in their migration to site of injury, proliferation and increased ECM synthesis in order to form an initial scar for reparation. Once reparation is completed, HSCs either return to its quiescent state or clear via apoptosis. The problem arises during chronic injury whereby persistent activation of HSCs causes continuous secretion of ECM components, leading to extensive scar formation termed as fibrosis and eventually hinders the function of liver. This review aims to provide updates on the emerging investigation on both physiological and pathological functions of HSCs as well as its therapeutic purposes in liver fibrosis.
Discussion
Physiological functions of HSCs in the normal liver
Most studies involving HSCs mainly focused on its fibrogenic properties in chronic liver diseases, and little attention was given to its function and identity. HSCs have been known to express CD133, a stem/progenitor cell marker and differentiate into endothelial cells [3]. Recent studies also showed the ability of HSCs to differentiate into adipocytes [4], osteocytes [4] and hepatocytes [5]. Although HSCs are unable to differentiate into blood cell lineages, HSCs were shown to support haematopoietic stem cells’ maintenance and development, hence supporting haematopoiesis [4]. Additionally, HSCs express mesenchymal stem cell (MSC)-related molecular markers and are, hence, liver-resident MSCs [4,6].
As for maintaining quiescence status of HSCs, it is reported that both vitamin A and insulin regulate quiescent HSC-associated genes including glial fibrillary acidic protein (GFAP), peroxisome proliferator-activated receptor-γ (PPAR-γ), CCAAT/enhancer-binding protein-α (C/EBP-α) and sterol regulatory element-binding protein 1 (SREBP-1) (see Table 1) [7]. The expression of these genes are modulated through vitamin A/Janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) pathway and insulin/SREBP-1 pathway [7]. A separate study demonstrated the ability of transmembrane protein 88 (TMEM88), a two-transmembrane protein found in HSCs, in inhibiting the activation and proliferation of HSCs by blocking Wnt/β-catenin pathway [8]. Its expression is regulated by DNA methyltransferase 3 alpha (DNMT3A) [8]. Furthermore, embryonic stem cell-expressed RAS (ERAS) protein, a member of the RAS family, was found to be expressed in quiescent HSCs but not in activated HSCs and other liver-resident cells [9]. By impeding proliferation of HSCs via Hippo signalling pathway and promoting cell survival via both phosphoinositide 3-kinase (PI3K)/phosphoinositide-dependent kinase-1 (PDK1) and mTOR Complex 2 (mTORC2) signalling pathways, ERAS prevents the transdifferentiation of HSCs into myofibroblasts [9].
Besides, there has been increasing evidence suggests the importance of microRNAs (miRNAs) in sustaining HSC quiescence [10,11]. MicroRNAs (miRNAs) are small non-coding RNAs consisting of about 22 nucleotides which act as regulators for post-transcriptional gene expression. A recent study demonstrated that miR-101 hinders the transforming growth factor-β (TGF-β)/SMAD pathway, one of the major fibrogenic signal transduction pathway, by suppressing the production of type I TGF-β receptor (TGFβRI) as well as a pro-fibrotic transcription factor known as Kruppel-like factor 6 (KLF6) [11]. In addition, miR-146a was also identified to be a suppressor of TGF-β/SMAD pathway by decreasing the common-partner SMAD4 expression directly and increasing the inhibitory SMAD7 expression through Wnt signalling pathway [12,13]. It was also recently shown that miR-146a is able to block toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) as well as TLR4/tumour necrosis factor receptor associated factor-6 (TRAF6)/c-Jun N-terminal kinases (JNK) signalling, resulting in reduced pro-fibrotic cytokines secretion and subsequent activation of HSCs [14]. Another study showed that up-regulation of miR-192 could reduce the expression of typical genes activated during liver fibrosis including α-smooth muscle actin (α-SMA), collagen 1 protein (COL1A1) and lysyl oxidase (LOX) which implicates its vital role in inhibiting the activation, migration and proliferation of HSCs [10]. These findings indicate that dysregulation of miRNAs is associated with the progression of liver fibrosis.
Pathological functions of HSCs during liver injury
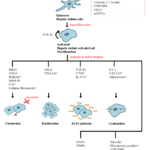
Initiation and perpetuation are two major phases of HSC activation while resolution phase will only occur when the injury subsides [15]. These phases are mediated by various stimulants (see Fig. 1).
Figure 1. Mediators involved in the various functions of HSCs. This schematic diagram highlights major mediators; new mediators are indicated with an asterisk (*) [7-14,25,30,36-40].
(i) Initiation phase
The initiation phase refers to early changes of HSCs both genetically and phenotypically, resulting in increased sensitivity of the cells towards stimulants. Initiation is mostly mediated through paracrine stimulation by neighbouring cells such as hepatocytes, macrophages and sinusoidal endothelial cells. Injured hepatocytes and apoptotic hepatocytes have been known to promote HSC initiation. One study demonstrated that hepatocyte nuclear factor 1 α (HNF1α) is downregulated in hepatocytes, leading to elevation of interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and TGF-β1 [16]. Besides, cell-to-cell vicinity and interaction between HSCs and hepatocytes is required for the activation of HSCs [16–18].
Macrophages can be either recruited from circulating monocytes or liver-resident macrophages (Kupffer cells). Both secrete pro-fibrotic mediators such as TGF-β1 and platelet-derived growth factor (PDGF). C-C motif chemokine receptor 9 positive (CCR9+) macrophages were shown to promote initiation of liver fibrosis by releasing TNF-α to activate HSC [19]. In addition, Kupffer cells also produce reactive oxygen species (ROS). The ROS family members that activate HSCs have been revealed to be negatively charged superoxide anion radicals (O2·–) and their influx is mediated by chloride channels [20].
(ii) Perpetuation phase
Perpetuation phase refers to the maintenance of HSC activated status and fibrosis generation. Perpetuation can be regulated either autocrinely or paracrinely and HSCs response to these stimulation with chemotaxis, proliferation, fibrogenesis and contraction.
After hepatic injury, chemokines are released triggering the migration of HSCs towards injured site. Typical HSC chemoattractants include monocyte chemotactic protein-1 (MCP-1), PDGF, vascular endothelial growth factor (VEGF) and osteopontin protein. Recent studies reported several other mediators responsible in HSC chemotaxis.
In alcohol-induced parenchymal cell injury, a nuclear protein known as high mobility group box 1 (HMGB1) is released from hepatocytes at site of injury and act as a chemokine, recruiting both HSCs and endothelial cells [21]. It was discovered that HMGB1 utilises both nonreceptor tyrosine kinase Src and extracellular signal-regulated kinase (ERK) for HSC migration [21]. Additionally, a recent research reported that macrophages produce matrix metalloproteinase-8 (MMP-8), a protease known to facilitate degradation of collagen and subsequent resolution, can be pro-fibrotic by promoting the migration of HSCs and increasing the expression of type 1 collagen [22]. The detailed mechanism, however, needs to be addressed in future studies.
Another chemotaxis regulator is complement component 5a (C5a) which is a complement peptides, bind to both C5a receptor (C5aR) and C5a anaphylatoxin chemotactic receptor (C5L2) on HSCs, stimulating multiple pathways and increase the mRNA expression of MCP-1 in activated HSCs [23]. Previously, cellular fibronectin secreted from sinusoidal endothelial cells was demonstrated to stimulate differentiation of HSCs to myofibroblasts [24], but a recent study found that it promotes chemotaxis of activated HSCs instead of myofibroblast differentiation [25]. Nevertheless, the molecular mechanism of cellular fibronectin in regulating HSC migration has yet to be defined.
PDGF is the most potent growth factor that stimulates proliferation of HSCs. It activates several intracellular signalling with mitogen-activated protein kinase (MAPK) being the major PDGF pathway. Recent studies provide updates on the regulatory mechanism of PDGF-specific HSC proliferation including the involvement of Ca2+/calmodulin-dependent protein kinase II (CaMKII) [26], transient receptor potential melastatin 7 (TRPM7) [27] and endosialin (CD248) [28,29] . They mainly target ERK, a type of signalling module in MAPK pathway [26,27,29].
TNF-related weak inducer of apoptosis (TWEAK), a TNF superfamily member, was also found to be a novel mitogen for HSCs [30]. TWEAK together with its receptor, fibroblast growth factor-inducible 14 (FN14), are co-expressed in HSCs [30]. They only promote HSC proliferation and do not intervene in differentiation and collagen synthesis activity of HSCs [30]. However, the molecular mechanism of its action remains to be clarified.
HSCs promote fibrosis by producing ECM components excessively. The space of Disse in healthy liver contains a low density basement-membrane-like matrix comprising of collagens type IV and VI [31]. This matrix is disrupted after liver injury and replaced by high density interstitial-like matrix composed of collagens type I and III [31]. Collagen type 1, a major ECM component, has been studied extensively [32–34].
TGF-β is the primary profibrogenic mediator responsible for stimulation of collagen production. Generally, TGF-β transmits intracellular signals by recruiting SMAD2 and SMAD3. They then form heterodimers with SMAD4 and translocate into nucleus where they increase transcription of collagen genes. Recently, it was reported that TRPM7 contributes to production of collagen [35]. TGF-β/SMAD signalling increases TRPM7 expression which in turn aid in phosphorylation of SMAD2 and SMAD3 [35]. Another study showed the involvement of interleukin-17A (IL-17A) produced by T helper cells [36]. Not only does IL-17A recruit macrophages, it also induces expression of TGF-β, increases TGF-β response by enhancing phosphorylation and nuclear translocation of SMAD2/3 as well as increases HSCs response towards TGF-β by up-regulating the expression of its receptor at the cell surface [36].
Wound contraction is an essential wound healing process. However, prolonged contraction will affect portal blood flow, resulting in increased portal resistance and eventually portal hypertension. Contractility of activated HSCs is known to be modulated by endothelin-1 (ET-1), which is a potent vasoactive protein. A recent study demonstrated that ET-1 expression is mediated by angiotensin (Ang)-II through Ang-II type 1 receptor (AT1) via PI3K/ protein kinase B (Akt) signalling pathway [37]. C-X-C motif chemokine 12 (CXCL12) was also reported to cause contraction of HSCs via C-X-C chemokine receptor type 4 (CXCR4) by activating the Rho kinase pathway [38]. Furthermore, aldosterone triggers the activation of RhoA/Rho-associated protein kinase (ROCK)-2 pathway, inducing HSC contraction [39].
(iii) Resolution phase
Fibrosis resolution refers to matrix degradation resulting from increased activity of collagenase mediated primarily by activated macrophages/Kupffer cells. This event requires either apoptosis of activated HSCs or reversion of myofibroblasts to quiescent HSCs. By removing the cellular source of ECM components, the accumulation could be controlled through ECM turnover or degradation and eventually reverting to healthy liver parenchyma.
Apoptosis of HSCs is normally induced by natural killer cells. A recent study proved that fibronectin peptides could also induce apoptosis in infiltrating monocyte/macrophages and HSCs by activating a signalling cascades with Src, inducible nitric oxide synthase (iNOS), JNK and p38 involvement [40]. They are actually fragmentised from fibronectin in ECM as a result of protease activity of MMPs such as MMP-9 [40]. In addition to suppressing HSC activation and proliferation, TMEM88 could also stimulate apoptosis of activated HSCs via B-cell lymphoma 2 (BCL-2)/BCL-2-associated X (BAX)/caspase-3 signalling [8].
Table 1. Features of quiescent, inactivated and activated HSCs .
↑ = Upregulated; ↓ = Downregulated; AdipoR1, adiponectin receptor 1; ADFP, adipose differentiation-related protein; BAMBI, BMP and activin membrane-bound inhibitor; C/EBP, CCAAT/enhancer-binding protein; DBP, D site of albumin promoter (albumin D-box) binding protein; GFAP, glial fibrillary acidic protein; INSIG1, insulin induced gene 1; LXR, liver X receptor; PPAR-γ, peroxisome proliferator-activated receptor-γ; SREBP-1c/SREBF1, sterol regulatory element-binding protein-1c/sterol regulatory element binding transcription factor-1; α-SMA/Acta2, α-smooth muscle actin/alpha-actin-2; Col1α1, type 1 collagen-α1; Col1α2, type 1 collagen-α2; Col2α1, type 2 collagen-α1; LOX, lysyl oxidase; TIMP1, tissue inhibitor of metalloproteinase; TGFβRI, type I transforming growth factor-β receptor.
Recently, several studies reported the reversion of activated HSCs to an inactive phenotype similar to that of quiescent HSCs with the etiological agent being removed [41–44]. Despite the fact that these reverted HSCs downregulate most of their fibrogenic genes expression and upregulate several quiescence HSC-related genes, they possess characteristics distinct from both quiescent and activated HSCs (see Table 1) [43]. These reverted HSCs are also found to be more susceptible to recurring fibrogenic stimulation such as stimulation by TGF-β [42,43].
Matrix remodelling is tightly regulated by MMPs which degrade ECM components and tissue inhibitor of matrix metalloproteinases (TIMPs) which inhibit the action of MMPs. MMPs are released by activated macrophages/Kupffer cells after the injury subsides. However, increased secretion of TIMPs by proliferating myofibroblast during chronic liver injury leads to imbalance between rates of matrix synthesis and degradation [31]. This imbalance will then progress to fibrosis because ECM becomes insoluble and resistant to degradation over time due to increased cross-linking between them [45].
Another mechanism is through angiogenesis, which is essential to provide nutrients and oxygen to the injured site by forming new blood vessels. VEGF, which is secreted from activated HSCs, plays a pivotal role in angiogenesis and was reported to facilitate the proliferation of hepatocytes during liver regeneration [46]. VEGF also facilitate fibrosis resolution by regulating vascular permeability, monocyte infiltration and function of scar-associated macrophage [47]. Its production is mediated autocrinely/paracrinely by prostaglandins such as PGD2 which are secreted from activated HSCs through the MAPK/cyclooxygenase-2 (COX-2) pathway [48,49]. The underlying mechanism is that prostaglandins stimulate hypoxia-inducible factor-1α (HIF-1α) synthesis, thereby degrading the negative regulator of VEGF production known as the von Hippel-Lindua protein [50].
HSCs in anti-fibrotic therapeutic strategies
To date, no effective therapeutic drugs are available for liver fibrosis in clinical practice. Recent studies suggested several potential anti-fibrotic agents targeting HSCs and they generally act by: (i) inhibiting proliferation of activated HSCs; (ii) inducing apoptosis of activated HSCs; and (iii) interfering formation of ECM components.
Dietary intake of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) is able to downregulate pro-fibrogenic genes expression in activated HSCs through proteasome-mediated degradation of yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) protein [51]. This in turn inhibits the proliferation and activation of HSCs as well as ECM deposition in hepatic fibrosis as YAP and TAZ are activated very early during HSC activation [52].
Melatonin, a natural hormone produced and secreted from pineal gland could also aid in ameliorating hepatic fibrosis by preventing the depletion of lipid droplets as well as proliferation and activation of HSCs [53]. With the absence of membranous melatonin receptors (MTRN1A and MTRN1B) in quiescent and activated HSCs, it acts directly on the nuclear melatonin receptor known as retinoic acid receptor-related orphan receptor-alpha (RORα/NR1F1) [53]. Melatonin’s anti-fibrotic action is demonstrated through suppression of RORα target gene Alox5 expression and consequently diminishes the production of 5-lipoxygenase (5-LO), which is a pro-inflammatory enzyme essential for leukotriene synthesis [53]. As a result, expression of COL1A1 and α-SMA are inhibited [53].
Cucurbitacin E (CuE) which is a compound isolated from cucurbitaceae plant was proposed to be a potential therapeutic agent for liver fibrosis [54]. It is able to attenuate fibrosis by preventing proliferation and inducing apoptosis of activated HSCs [54]. Apoptosis is possible using CuE by blocking PI3K/Akt signalling, resulting in increased AMP-activated protein kinase (AMPK) activity which in turn suppresses mechanistic target of rapamycin (mTOR) pathway [54]. In addition, CuE also reduces the expressions of α-SMA, TIMP-1 and collagen 1 protein [54].
Besides promoting motility of HSCs, fibronectin is necessary for collagen matrix formation and maintenance of matrix integrity [55–57]. Despite the collagen assembling ability of HSCs without fibronectin [58], intervening fibronectin and thus deposition of collagen by a fibronectin inhibitory peptide is sufficient to reduce hepatic fibrosis by decreasing accumulation of fibronectin and type I collagen [59].
Conclusion
There have been emerging advances in the investigation of both cellular and molecular mechanism of liver fibrosis. Nonetheless, more concrete evidence of the reversion of activated HSCs to quiescent HSCs are required. Future work to unravel the regulatory mechanism of TGF-β/SMAD signalling pathway is also very important owing to the fact that it stimulate HSC fibrogenesis.
Given such continuous endeavour in clarifying the underlying mechanisms of liver fibrosis as well as the increasing evidence of fibrosis reversibility, the field has slowly progressed towards translating these important findings into the development of anti-fibrotic therapies that are both effective and safe to be used in human. These efforts may eventually stop the progression of liver fibrosis in the near future.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank the International Medical University, Malaysia for supporting this work. No funding has been involved in the preparation and submission of this manuscript.
References
- Mederacke I, Hsu CC, Troeger JS, et al. Nat Commun. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. 2013;4:2823.
- Puche JE, Lee YA, Jiao J, et al. Hepatology. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. 2013;57(1):339–50.
- Kordes C, Sawitza I, Müller-Marbach A, et al. Biochem Biophys Res Commun. CD133+ hepatic stellate cells are progenitor cells. 2007;352(2):410–7.
- Kordes C, Sawitza I, Götze S, Häussinger D. Cell Physiol Biochem. Hepatic stellate cells support hematopoiesis and are liver-resident mesenchymal stem cells. 2013;31(2–3):290–304.
- Kordes C, Sawitza I, Götze S, Herebian D, Häussinger D. J Clin Invest. Hepatic stellate cells contribute to progenitor cells and liver regeneration. 2014;124(12):5503–15.
- Castilho-Fernandes A, de Almeida DC, Fontes AM, et al. Exp Mol Pathol. Human hepatic stellate cell line (LX-2) exhibits characteristics of bone marrow-derived mesenchymal stem cells. 2011;91(3):664–72.
- Yoneda A, Sakai-Sawada K, Niitsu Y, Tamura Y. Exp Cell Res. Vitamin A and insulin are required for the maintenance of hepatic stellate cell quiescence. 2016;341(1):8–17.
- Cai S-P, Cheng X-Y, Chen P-J, et al. Mol Immunol. Transmembrane protein 88 attenuates liver fibrosis by promoting apoptosis and reversion of activated hepatic stellate cells. 2016;80:58–67.
- Nakhaei-Rad S, Nakhaeizadeh H, Götze S, et al. J Biol Chem. The role of embryonic stem cell-expressed ras (eras) in the maintenance of quiescent hepatic stellate cells. 2016;291(16):8399–413.
- Coll M, Taghdouini A El, Perea L, et al. Sci Rep. Integrative miRNA and gene expression profiling analysis of human quiescent hepatic stellate cells. 2015;5:11549.
- Tu X, Zhang H, Zhang J, et al. J Pathol. MicroRNA-101 suppresses liver fibrosis by targeting the TGFβ signalling pathway. 2014;234(1):46–59.
- He Y, Huang C, Sun X, Long X ran, Lv X wen, Li J. Cell Signal. MicroRNA-146a modulates TGF-β1-induced hepatic stellate cell proliferation by targeting SMAD4. 2012;24(10):1923–30.
- Du J, Niu X, Wang Y, et al. Sci Rep. MiR-146a-5p suppresses activation and proliferation of hepatic stellate cells in nonalcoholic fibrosing steatohepatitis through directly targeting Wnt1 and Wnt5a. 2015;5:16163.
- Chen Y, Zeng Z, Shen X, Wu Z, Dong Y, Cheng J. Int J Mol Sci. MicroRNA-146a-5p negatively regulates pro-inflammatory cytokine secretion and cell activation in lipopolysaccharide stimulated human hepatic stellate cells through inhibition of toll-like receptor 4 signaling pathways. 2016;17(7):1076.
- Friedman SL. Physiol Rev. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. 2008;88(1):125–72.
- Qian H, Deng X, Huang Z-W, et al. Cell Res. An HNF1α-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells. 2015;25(8):930–45.
- Giraudi PJ, Barbero Becerra VJ, Marin V, Chavez-Tapia NC, Tiribelli C, Rosso N. Exp Mol Pathol. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. 2015;98(1):85–92.
- Barbero-Becerra VJ, Giraudi PJ, Chávez-Tapia NC, Uribe M, Tiribelli C, Rosso N. Toxicol Vitr. The interplay between hepatic stellate cells and hepatocytes in an in vitro model of NASH. 2015;29(7):1753–8.
- Chu P, Nakamoto N, Ebinuma H, et al. Hepatology. C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice. 2013;58(1):337–50.
- den Hartog GJM, Qi S, van Tilburg JHO, Koek GH, Bast A. Eur J Pharmacol. Superoxide anion radicals activate hepatic stellate cells after entry through chloride channels: A new target in liver fibrosis. 2014;724:140–4.
- Seo YS, Kwon JH, Yaqoob U, et al. AJP Gastrointest Liver Physiol. HMGB1 recruits hepatic stellate cells and liver endothelial cells to sites of ethanol-induced parenchymal cell injury. 2013;305(11):G838–48.
- Baig MS, Yaqoob U, Cao S, Saqib U, Shah VH. Life Sci. Non-canonical role of matrix metalloprotease (MMP) in activation and migration of hepatic stellate cells (HSCs). 2016;155:155–60.
- Das D, Barnes MA, Nagy LE. Fibrogenesis Tissue Repair. Anaphylatoxin C5a modulates hepatic stellate cell migration. 2014;7(1):9.
- Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. J Cell Biol. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. 1994;127(6 Pt 2):2037–48.
- Olsen AL, Sackey BK, Marcinkiewicz C, Boettiger D, Wells RG. Gastroenterology. Fibronectin extra domain-a promotes hepatic stellate cell motility but not differentiation into myofibroblasts. 2012;142(4):928–937.e3.
- Ping A, Yihao T, Jingxing D, Minkai C, Hesheng L. Dig Dis Sci. Ca2+/calmodulin-dependent protein kinase ii mediates platelet-derived growth factor-induced human hepatic stellate cell proliferation. 2012;57(4):935–42.
- Fang L, Zhan S, Huang C, et al. Toxicol Appl Pharmacol. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. 2013;272(3):713–25.
- Mogler C, Wieland M, Konig C, et al. EMBO Mol Med. Hepatic stellate cell-expressed endosialin balances fibrogenesis and hepatocyte proliferation during liver damage. 2015;7(3):332–8.
- Wilhelm A, Aldridge V, Haldar D, et al. Gut. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism. 2016;65(7):1175–85.
- Wilhelm A, Shepherd EL, Amatucci A, et al. J Pathol. Interaction of TWEAK with FN14 leads to the progression of fibrotic liver disease by directly modulating hepatic stellate cell proliferation. 2016;239(1):109–21.
- Hernandez-Gea V, Friedman SL. Annu Rev Pathol Mech Dis. Pathogenesis of liver fibrosis. 2011;6(1):425–56.
- Xiang X, Jiang T, Zhang S, et al. Mol Med Rep. Pirfenidone inhibits proliferation, arrests the cell cycle, and downregulates heat shock protein-47 and collagen type I in rat hepatic stellate cells in vitro. 2015;12(1):309-14.
- Du M, Zhang J, Xu D, Li W, Liu J, Liu F. Mol Med Rep. Inhibition of pro‑collagen I expression by oxymatrine in hepatic stellate cells is mediated via nuclear translocation of Y‑box binding protein 1. 2015;12(6):8101–6.
- de Galarreta MR, Navarro A, Ansorena E, et al. Biochim Biophys Acta – Mol Cell Res. Unfolded protein response induced by Brefeldin A increases collagen type I levels in hepatic stellate cells through an IRE1α, p38 MAPK and SMAD-dependent pathway. 2016;1863(8):2115–23.
- Fang L, Huang C, Meng X, et al. Toxicol Appl Pharmacol. TGF-β1-elevated TRPM7 channel regulates collagen expression in hepatic stellate cells via TGF-β1/SMAD pathway. 2014;280(2):335–44.
- Fabre T, Kared H, Friedman SL, Shoukry NH. J Immunol. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-receptor on hepatic stellate cells in a JNK-dependent manner. 2014;193(8):3925–33.
- He C, Miao X, Li J, Qi H. Dig Dis Sci. Angiotensin II induces endothelin-1 expression in human hepatic stellate cells. 2013;58(9):2542–9.
- Saiman Y, Agarwal R, Hickman DA, et al. AJP Gastrointest Liver Physiol. CXCL12 induces hepatic stellate cell contraction through a calcium-independent pathway. 2013;305(5):G375–82.
- Ji H, Meng Y, Zhang X, et al. Regul Pept. Aldosterone induction of hepatic stellate cell contraction through activation of RhoA/ROCK-2 signaling pathway. 2011;169(1–3):13–20.
- Mòdol T, Brice N, Ruiz de Galarreta M, et al. J Cell Physiol. Fibronectin peptides as potential regulators of hepatic fibrosis through apoptosis of hepatic stellate cells. 2015;230(3):546–53.
- She H, Xiong S, Hazra S, Tsukamoto H. J Biol Chem. Adipogenic transcriptional regulation of hepatic stellate cells. 2005;280(6):4959–67.
- Troeger JS, Mederacke I, Gwak G-Y, et al. Gastroenterology. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. 2012;143(4):1073–83.e22.
- Kisseleva T, Cong M, Paik Y, et al. Proc Natl Acad Sci USA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. 2012;109(24):9448–53.
- El Taghdouini A, Najimi M, Sancho-Bru P, Sokal E, van Grunsven LA. Fibrogenesis Tissue Repair. In vitro reversion of activated primary human hepatic stellate cells. 2015;8(1):14.
- Issa R, Zhou X, Constandinou CM, et al. Gastroenterology. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. 2004;126(7):1795–808.
- Tekkesin N, Taga Y, Sav A, Almaata I, Ibrisim D. Hepatogastroenterology. Induction of HGF and VEGF in hepatic regeneration after hepatotoxin-induced cirrhosis in mice. 2011;58(107–108):971–9.
- Yang L, Kwon J, Popov Y, et al. Gastroenterology. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. 2014;146(5):1339–1350.e1.
- Zhao Y, Wang Y, Wang Q, Liu Z, Liu Q, Deng X. Mol Cell Biochem. Hepatic stellate cells produce vascular endothelial growth factor via phospho-p44/42 mitogen–activated protein kinase/cyclooxygenase-2 pathway. 2012;359(1–2):217–23.
- Paiva LA, Coelho KA, Luna-Gomes T, et al. Prostaglandins, Leukot and Essent Fat Acids. Schistosome infection-derived hepatic stellate cells are cellular source of prostaglandin D2: Role in TGF-β-stimulated VEGF production. 2015;95:57–62.
- Wang YQ, Luk JM, Ikeda K, et al. Biochem Biophys Res Commun. Regulatory role of vHL/HIF-1α in hypoxia-induced VEGF production in hepatic stellate cells. 2004;317(2):358–62.
- Zhang K, Chang Y, Shi Z, et al. Sci Rep. ω-3 PUFAs ameliorate liver fibrosis and inhibit hepatic stellate cells proliferation and activation by promoting YAP/TAZ degradation. 2016;6:30029.
- Mannaerts I, Leite SB, Verhulst S, et al. J Hepatol. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. 2015;63(3):679–88.
- Shajari S, Laliena A, Heegsma J, Tuñón MJ, Moshage H, Faber KN. J Pineal Res. Melatonin suppresses activation of hepatic stellate cells through ROR α -mediated inhibition of 5-lipoxygenase. 2015;59(3):391–401.
- Wu Y-L, Zhang Y-J, Yao Y-L, et al. Toxicol Lett. Cucurbitacin E ameliorates hepatic fibrosis in vivo and in vitro through activation of AMPK and blocking mTOR-dependent signaling pathway. 2016;258:147–58.
- Sottile J. Mol Biol Cell. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. 2002;13(10):3546–59.
- Velling T. J Biol Chem. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins α11β1and α2β1. 2002;277(40):37377–81.
- Bentmann A, Kawelke N, Moss D, et al. J Bone Miner Res. Circulating fibronectin affects bone matrix while osteoblast fibronectin modulates osteoblast function. 2010; 25(4):706-15.
- Moriya K, Bae E, Honda K, et al. Gastroenterology. A Fibronectin-independent mechanism of collagen fibrillogenesis in adult liver remodeling. 2011;140(5):1653–63.
- Altrock E, Sens C, Wuerfel C, et al. J Hepatol. Inhibition of fibronectin deposition improves experimental liver fibrosis. 2015;62(3):625–33.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/