J Med Discov (2023); 8(1):jmd23020; DOI:10.24262/jmd.8.1.23020; Received May 2nd, 2023, Revised August 8th, 2023, Accepted September 4th, 2023 , Published September 10th, 2023.
Two case reports and literature review of refractory salivary gland (epithelial) myoepithelial carcinoma with pathological transformation
Chao Peng#1,2, Shumin Cao#1,3, Yimeng Qian1,4,Lin Kang5, Xin Huang1, Jing Zhao✳1
1Department of Oncology, Hebei General Hospital, Shijiazhuang, Hebei, China;
2Graduate School, North China University of Science and Technology, Tangshan, Hebei, China;
3Graduate School, Hebei Medical University, Shijiazhuang, Hebei, China;
4Graduate School, Hebei North University, Zhangjiakou, Hebei, China;
5Department of Pathology, Hebei General Hospital, Shijiazhuang, Hebei, China;
* Correspondence:Jing Zhao,Department of Oncology, Hebei General Hospital, Shijiazhuang, Hebei, China. Email:zjzzmail@163.com.
Abstract
Objective: To investigate the clinicopathologic features of parotid myoepithelial carcinoma and epithelial-myoepithelial carcinoma, to improve the understanding of pathological type transformation of parotid myoepithelial carcinoma and epithelial-myoepithelial carcinoma, and to provide possible effective treatment strategies for refractory parotid myoepithelial carcinoma and epithelial-myoepithelial carcinoma.
Methods: The clinical data, imaging features and pathological features of 2 patients with parotid myoepithelial carcinoma and epithelial-myoepithelial carcinoma admitted to the undergraduate department were retrospectively analyzed, and the treatment experience was summarized, and the relevant literature was reviewed.
Results: The first patient received surgery, radiotherapy, radioactive particle implantation, chemotherapy and targeted therapy, but the disease still progressed, and died of lung metastasis and lung infection 5 years after diagnosis, with an OS of 5 years. The second patient received local chemoradiotherapy but progressed and finally achieved partial remission (PR) after changing his treatment regimen to immunotherapy combined with chemotherapy. Patients were followed up for more than 2 years without recurrence, metastasis or treatment-related adverse reactions, and PFS were 2.8 years.
Conclusion: The increased malignancy caused by pathological transformation may be one of the reasons for the recurrence, metastasis, drug resistance and poor prognosis of salivary gland myoepithelial carcinoma and epithelial-myoepithelial carcinoma. Therefore, after disease progression, another pathological examination is necessary. In addition, immunotherapy combined with chemotherapy has a promising application prospect in the treatment of refractory parotid myoepithelial carcinoma and epithelial-myoepithelial carcinoma.
Keywords:myoepithelial carcinoma, epithelial-myoepithelial carcinoma, refractory, pathological transformation, immunotherapy
1. Introduction
Myoepithelial carcinomas (MCs) and epithelial-myoepithelial carcinomas (EMCs) are clinically rare, low-grade malignant tumors, mainly occurring in the salivary gland, each accounting for about 1% of salivary gland tumors.[1, 2] Domestic and foreign studies on the disease are mostly case reports, among which cases of the transformation of pathological types are extremely rare. We reported two cases of salivary gland MC and EMC with pathological transformation during disease progression and review the literature (Table 1) to summarize the clinical manifestations, imaging, histopathological, treatment features, and prognosis to improve the understanding of salivary gland MC and EMC with pathological type transformation and provide a potentially effective treatment strategy for refractory salivary gland MC and EMC.
Table 1 A list of literature reviews on EMC/MC with pathological type transformation
| Year | Author | Age | Sex | Tumor Site | Original tumor | Pathological-transformed tumor | Morphology after transformation | Immunohistochemical results | Outcome(m) |
| 1999 | Alos[7] | 88 | F | Parotid | EMC | Adenocarcinoma | The cells are large with vesicular and pleomorphic nuclei showing prominent nucleoli | Cytokeratins (+), CAM5.2(+), EMA (+), CEA (+), MSA (-), SMA (f+), S100 (-), Vimentin (-), Desmin (-), GFAP (-) | DOD (36) |
| 78 | M | Parotid | EMC | Undifferentiated large cell carcinoma | large cells with pleomorphic and atypical nuclei | Cytokeratins (-), CAM5.2 (-), EMA (-), CEA (-), MSA (-), SMA (-), S100 (-), Vimentin (-), Desmin (-), GFAP (-) | NED (72) | ||
| 66 | F | Palate | EMC | Adenocarcinoma | large cells with vesicular nuclei and patent nucleoli, have a tendency to glands formation. | Cytokeratins (+), CAM5.2 (+), EMA (f+), CEA (f+), MSA (-), SMA (-), S100 (f+), Vimentin (-), Desmin (-), GFAP (-) | NED (7) | ||
| 2000 | Fonseca[8] | 69 | M |
Parotid
|
EMC | Undifferentiated large cell carcinoma | nuclear enlargement and pleomorphism | CAM5.2 (+),34βE12 (+), HHF35(+), S100(+), cerbB2(+), P53(+), | N/A |
| 2003 | Ogawa[9] | 59 | M | Left preauricular region | MC | Undifferentiated carcinoma | Anaplastic spindle cells growing in a fascicular arrangement | AE1/AE3(f+), Vimentin (+), S100(-), GFAP (-), SMA (-), P53(+), CyclinD1(+) | AWD (18) |
| 2010 | Roy[10] | 82 | M | Parotid | EMC | N/A | Clear cells, focal squamous pearls | N/A | N/A |
| 74 | F | Parotid | EMC | high-grade adenocarcinoma | 1. High-grade ca with focal ducts;2. High-grade ca with clear cells, spindling, and focal squamous pearls | LMWK (ducts+); S100(Patchy+), CK14, HMWK (squamous areas+) | NED (24) | ||
| 59 | F | Parotid | EMC | N/A | High-grade ca with plasmacytoid cells | p63+only | AWD (36) | ||
| 2014 | AydJn[11] | 46 | F | Parotid | EMC | poorly differentiated adenocarcinoma | solid structure and rare glandular structures | SMA (+), P63(+), CK8(+), S100(+) | NED (36) |
| 2017 | BoWen Li[12] | 77 | F | Parotid | EMC | Pleomorphic sarcoma | intensive spindle cells with increased nuclear pleomorphism, mitotic activity, prominent nucleolus and acidophilic cytoplasm | All stains negative | NED (48) |
| 2020 | Yuichiro[13] | 71 | M | right parotid | EMC | SDC | irregularly shaped large nucleus and swollen nucleoli and abundant eosinophilic cytoplasm | AR (+), GCDFP15(f+), HER2(+), AE1/AE3(+), EMA (+), P63(-), P40(-), SMA (-), S100(-), GFAP (-), DOG1(-), WT1(-), Ki67(30%) | DOD (19) |
| 2021 | Yanagawa[14] | 62 | M | right parotid | EMC/MC | SDC | The cell type was atypical with abundant gran-ular eosinophilic cytoplasm, large nuclei with coarse chromatin and prominent nucleoli. The cells were proliferative and invasive with duct-like, glandular and solid patterns | AR (+), HER2(+), AE1/AE3(+), EMA (+), P63(-), SMA (-), S100(-), WT1(-), P53(+), Ki67(50%) | N/A |
AWD, alive with disease; DOD, dead of disease; NED, no evidence of disease.
2. Case Report
2.1. Ethic and informed consent
This cases report was approved by the institutional review board of the Hebei General Hospital. Informed consent was obtained from the patients for publication of this cases report and accompanying images.
2.2. Case presentation
Case 1. An 85-year-old woman observed a tumor in her left parotid gland area in June 2017. Computed tomography (CT) of the left parotid gland showed cystic solid space-occupying lesions but no abnormally enlarged lymph nodes in the neck (Fig.1). So the patient underwent a left parotid mass and partial gland resection. The postoperative pathological reported MC. Immunohistochemical staining showed CKpan(+), vimentin(+), epithelial membrane antigen (EMA)(+), S100(+), smooth muscle myosin heavy chain (SMMHC)(±), smooth muscle actin (SMA)(±), P63 (partial cells+), human epidermal growth factor receptor 2 (HER2)(+), CK17 (scattered few cells+) ,P40(-), CK7(-), adrogen receptor (AR)(-), Syn(-), CD5(-), CD56(-), CD117(-), CD21(-), and a Ki-67 positivity rate of approximately 60%. Alcian blue/periodic acid–Schiff (AB/PAS) staining was negative (Fig.2). Thus, the patient was diagnosed with MC (T2N0M0) of the left parotid region. Three months after the operation, The patient developed another mass in the left parotid region, which gradually grew to the size of “egg”, and the pain was obvious. A fine needle puncture was performed on the left parotid gland to indicate the discovery of cancer cells. So local radiotherapy was performed with a planning target volume (PTV) of 60 Gy/30 F and a gross tumor volume (GTV) of 63.9 Gy/30 F. Reexamination at the end of treatment showed that the mass in the left parotid region was reduced, and the efficacy was evaluated as partial remission (PR). Regular examinations following this treatment showed no signs of recurrence or metastasis until the patient presented with a mass behind her left ear in July 2021, which progressively enlarged in size and was accompanied by pain, edema, and numbness of the left side of her face. The corner of her mouth was also skewed to the right. The patient was hospitalized in our department. The physical examination showed a body mass index (BMI) of 20.05 kg/m2, body surface area (BSA) of 1.24 m2, bulging cheeks, and air leakage. Her left frontal crease and left nasolabial groove were shallower, her mouth was askew to the right, and a hard, fixed, ill-defined, and tender nodule about 30 mm×20 mm in size was palpated behind her left ear. Head and neck CT showed left parotid carcinoma with a high possibility of recurrence after surgery. The lesion reached the parapharyngeal space inward and the left oval foramen upward, with a cross-sectional area of approximately 46 mm×18 mm (Fig.3A). Slightly larger submental lymph nodes (Fig.3B). Chest CT showed multiple nodules of different sizes in both lungs; thus, metastasis was considered (Fig.3C). A bone scan showed localization of the imaging agent in the frontal bone, with increased intramedullary density suggestive of bone metastases. The patient had no surgical indications and could not tolerate a second course of radiotherapy. Therefore, she was administered palliative radioactive I-125 seed implantation for left parotid gland cancer and left parotid gland tumor biopsy under CT and ultrasound guidance. During the procedure, 77, 0.5-mci particles were implanted into the left parotid gland tumor (Fig.3D). Puncture histopathological examination showed a malignant tumor of salivary gland origin. Combined with the findings of immunohistochemical staining, the tumor was considered an acinar cell carcinoma. The immunohistochemical staining showed Ckpan(+), P53(-), vimentin(-), CK7(-), SMA(-), S100(-), calponin(-), SOX-10(+), mammaglobin (-), DOG-1(+), CD5/6(scattered+), AR(-), and approximately 50% Ki-67 positivity (Fig. 4). Gene detection in the tissue showed microsatellite stable (MSS), negativity for the HER2, neurotrophic tyrosine receptor kinase (NTRK), and other driver genes, as well as programmed death-ligand 1 (PD-L1) (DAKO 22C3). After surgery, targeted therapy with Nimotuzumab (200mg, d1, q1W) and paclitaxel (300mg, d2, q3W) were given. After treatment, head and neck CT showed that the submental lymph node was significantly larger than before; thus, lymph node metastasis was considered (Fig.3E). Chest CT showed high potential for multiple metastases in both lungs (Fig.3F). The right pedicle of T1 also showed rounded high density. Thus, bone metastasis could not be excluded. Efficacy evaluation showed progressive disease (PD). The patient refused to continue treatment. Through follow-up, we learned that she died of lung metastasis and lung infection in June 2022. The OS was 5 years. The OS before pathological transformation was 4 years, and the OS after pathological transformation was 5 years.
Fig. 1 CT of parotid gland of case 1.
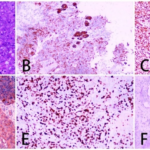
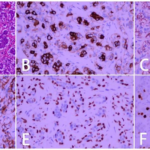
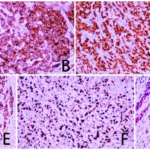
Fig. 2 Postoperative pathological examination results of case 1. (A: HE×200; B: CKpan (+); C: Vimentin (+); D: SMA (+); E: Ki-67 (60%+); F: CK7 (-).)
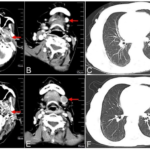
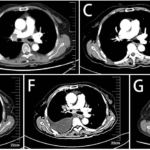
Fig. 3 Head and neck CT and Chest CT before and after particle implantation of case 1. (A: Before particle implantation; B: Anterior submental lymph node enlargement after particle implantation and targeted treatment; C: Lung metastasis after particle implantation and before targeted therapy; D: after particle implantation; E: After 2 cycles of targeted therapy, submental enlarged lymph nodes were significantly larger than before treatment; F: After 2 cycle targeted therapy, lung metastases were significantly larger than before treatment.)
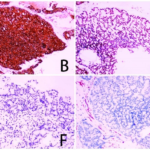
Fig. 4 Secondary pathological examination results of case 1. (A: HE×200; B: CKpan (+); C: SOX-10 (+); D: Ki-67 (50%+); E: DOG-1 (+); F: P63 (-); G: Vimentin (-); H: SMA (-).)
Case 2. A 66-year-old woman was hospitalized in March, 2020, mainly due to progressive dysphagia for 6 months and left facial numbness for more than 2 months. Examination showed a BMI of 22.19 kg/m2, BSA of 1.5 m2. CT of the nasopharynx revealed a left parapharyngeal mass (Fig.5A). Biopsy of the mass behind the left parotid gland and immunohistochemistry, suggested EMC. The immunohistochemical staining showed P63(myoepithelial+), P40(myoepithelial+), CK5/6(myoepithelial+), CKpan(+), vimentin(-), S100(myoepithelial+), glial fibrillary acidic protein (GFAP)(myoepithelial+), desmin(-), SMA(+), CD117(weak+), >80% Ki-67 positivity,
and CK7(+)(Fig.6). The tumor was diagnosed as a parotid EMC (T4aN2M0). The patient was administered radiotherapy. The total dose (DT) was 70 Gy/35 F and docetaxel (120 mg, d1, q3W) chemotherapy were administered. Five cycles, the efficacy evaluation was stable disease (SD). Then the replacement plan was paclitaxel (300 mg, d1, q3W) chemotherapy for two cycles. The efficacy evaluation is SD. In March, 2021, positron emission tomography (PET)/CT revealed that the patient was stable. Reexamination CT showed neck recurrence and lung metastasis in June 2021, and anrotinib (12mg, d1-14, q3w) was used. PET/CT reexamination in August, 2021, showed no abnormal metabolism in the left parotid gland area; however, an irregular nodule with high metabolism was observed in the left lower lobe interlobar fissure, suggesting lung metastasis. Multiple lymph nodes with hypermetabolism were observed in area IV, area V of the left neck, the 4R and area 7of mediastinum, and the bilateral hilus of the lung, suggesting lymph node metastasis. Hypermetabolism in the second thoracic vertebra, the left part of the first sacral vertebra, and the sclerotin of the upper part of the right femur bone suggested bone metastases, indicating disease progression (Fig.5B, Fig.7A). Chest CT showed multiple enlarged lymph nodes in the mediastinal and bilateral hilar areas, and metastasis was considered (Fig.7B). The patient had an operation on the left neck mass under local anesthesia in August, 2021. The postoperative pathological results revealed ductal carcinoma (DC) of the salivary gland. The immunohistochemical staining results were: CK7(+), CK34βE12(+), GCDFP-15(+), AR(+), SMA(-), S100(-), GFAP(-), calponin(-), P63(scattered+), CEA(-), ER(-), PR(-), PSA(-), and 40%+ Ki-67 positivity (Fig.8). Genetic testing showed MSS and HER2 amplification, and PD-L1 CPS (DAKO 22C3) =1, positivity. Considering that the local neck mass was due to parotid carcinoma metastasis, the patient was given four cycles of pembrolizumab (200mg, d1, q3W) immunotherapy combined with tegafur (60mg, d1-14, q3W) chemotherapy. Chest CT in January 2022, showed that the small oval soft tissue density nodules near the hilum in the left interlobar fissure and multiple small lymph nodes in the mediastinum and bilateral hilus of lung areas were significantly smaller than before (Fig.7C). The efficacy evaluation was PR. Three cycles of pembrolizumab immunotherapy combined with tegafur chemotherapy were continued. Chest CT in April 2022, showed that the small oval soft tissue density nodules near the hilum in the left interlobar fissure and multiple small lymph nodes in the mediastinum and bilateral hilus of lung areas were significantly smaller than before (Fig.7D). Maintain PR. Then two cycles of pembrolizumab immunotherapy combined with tegafur chemotherapy were continued. In July 2022, PET/CT indicated that lymph nodes in the left supraclavicular, mediastinum and hilar were consistent with intrapulmonary and multiple lymph node metastases, which were more numerous and larger than before. Chest CT showed multiple nodules in both lungs, some of which were new and some of which were larger than before. Considering metastasis. Multiple enlarged lymph nodes in the mediastinum and bilateral hilar area were partially fused into clusters and some of which were larger than before (Fig.7E). PET/CT and chest CT indicated the progression of the disease, so the treatment regimen was changed to pembrolizumab immunotherapy combined with gemcitabine chemotherapy. Reexamination of chest CT in October 2022 showed multiple metastatic tumors in both lungs, which were less and smaller than before, multiple enlarged lymph nodes in the mediastinum and bilateral hilar area, which were smaller than before (Fig.7F). The efficacy evaluation was PR after three cycles. Reexamination of chest CT in January 2023 showed multiple metastatic tumors in both lungs, which were less and smaller than before, multiple enlarged lymph nodes in the mediastinum and bilateral hilar area, which were smaller than before (Fig.7G). Maintain PR. At present, pembrolizumab immunotherapy combined with gemcitabine chemotherapy has been performed for 6 cycles, and the efficacy maintains PR. Through follow-up and review, we evaluated that the patient currently maintained PR and PFS for 2.8 years.
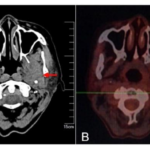
Fig. 5 Nasopharyngeal CT before treatment and PET/CT after radiotherapy and chemotherapy of case 2. (A: nasopharyngeal CT before treatment;B: PET/CT after chemoradiotherapy.)
Fig. 6 The results of primary pathology of case 2. (A: HE×200; B: CK7 (+); C: Vimentin (+); D: SMA (Myoepithelial+); E: P63 (Myoepithelial+); F: Ki-67 (>80%+).)
Fig. 7 PET/CT and chest CT of case 2 (A: PET/CT in 2021-8;B: Chest CT in 2021-8;C: Chest CT in 2022-1;D: Chest CT in 2022-4;E: Chest CT in 2022-7;F: Chest CT in 2022-10;G: Chest CT in 2023-1)
Fig. 8 Secondary pathological examination results of case 2. (A: HE×200; B: CK34βE12(+); C: AR (+); D: GCDFP-15(+); E: CK7(+); F: Ki-67(40%+); G: SMA (-).)
3. Discussion
3.1 Epidemiological and clinical characteristics
MC and EMC are extremely rare clinically and are categorized as low-grade salivary gland tumors.[1, 2] MC accounts for about 1% of salivary gland malignancies and occurs slightly more often in women compared to men.[1] EMC is a malignant tumor involving both glandular and myoepithelial tumors that accounts for <2% of salivary gland tumors, and also occurs slightly more often in women compared to men.[2] Both MC and EMC mainly occur in the parotid gland, followed by the submandibular gland, and the age of onset is more common in the middle-aged and elderly.[2, 3] Most cases show a chronic course, and the clinical manifestations are mainly parotid gland masses of different sizes, most of which are approximately 4 cm in diameter. Some patients may have clinical manifestations such as limited mouth opening, pain in the mass area, and facial paralysis. Some tumors have accelerated growth and, as the tumor grows, the surface may rupture.[2, 3] Some patients are admitted with regional lymph-nodes metastases or distant metastases in the bone, liver, and lung.[2, 3] The two cases reported here were both elderly women, both of whom presented with a mass in the parotid gland area on one side and symptoms of facial nerve invasion, as well as distant metastasis to the lymph nodes and lungs. These clinical features were consistent with those of parotid MC and EMC described above.
3.2 Pathological features and immunohistochemistry
Pathological examination is the “gold standard” for the diagnosis of MC and EMC, and should to combined with immunohistochemical staining. Myoepithelial markers such as CK, S100, SMA, etc., are positive to varying degrees.[1] A Ki-67 index >10% can inform the diagnosis of MC.[3] Some studies have reported high p53 protein expression in MC.[3] EMC cells have a typical double-layered casing-like structure, which is very characteristic and part of the basis for the diagnosis of this tumor by HE staining.[2] Immunohistochemical analysis shows positivity for SMA, P63, actin, myosin, and S100.[2] A Ki-67 index >10% is also helpful for the diagnosis of EMC. For cases of suspected MC and EMC, multiple myoepithelial marker antibodies should be assessed. Positivity for the epithelial marker CK, breast duct marker GCDFP-15, and sex hormone marker AR can prompt the diagnosis of DC.[4] In acinar cell carcinoma (ACC), in addition to CKpan, positivity for SOX-10 and DOG proteins can inform diagnosis.[5] Of the two cases reported here, Case1 was initially diagnosed with salivary MC based on the combined findings of the pathological examination and immunohistochemistry. The initial pathological examination of Case 2 showed a typical double-sleeve-like structure. Combined with positivity for the glandular epithelium and myoepithelial markers CK, P63, SMA, S100, etc., the diagnosis was salivary EMC. However, due to repeated recurrence and poor treatment effect, pathological type transformation was identified in the second pathological biopsy. Acinar components were observed in the pathological tissue of Case 1, and immunohistochemical staining was positive for SOX-10 and DOG, revealing that the MC had transformed into ACC. Ductal components in the pathological tissue of Case 2, along with immunohistochemical staining showed positivity for CK34βE12, GCDFP-15, and AR, suggested that the EMC had transformed into salivary duct carcinoma (SDC). The transformation of pathological types in both cases indicated an increase in the degree of malignancy. Among them, SDC is a highly aggressive tumor, suggesting the need for repeat biopsy in cases with refractory salivary gland MCs and EMCs with repeated recurrence or poor curative effect and continuous progression.
3.3 Genetics
Genetic research on MC and EMC is limited at present. Previous studies have shown that HER2 is not expressed in benign salivary gland tumors, but that different HER2 expression levels can occur in salivary gland malignant tumors, especially DC and MC, which often show high expression.[6] The second pathological biopsy of Case 2 in the present report showed a transformation of pathological type from EMC to SDC. Genetic testing showed HER2 amplification. This result was consistent with the above report. Genetic testing in Case 1 did not show high HER2 expression.
3.4 Treatment and prognosis
Parotid MCs and EMCs are low-grade malignant cancers; however, However, the study found that the lymph node metastasis rate can reach up to 41%, the distant metastasis rate can reach 47%, and the recurrence rate can reach 51.9%, which shows that they are highly prone to recurrence and metastasis, resulting in a poor prognosis.[3] At present, the treatment is mainly surgery, supplemented by radiotherapy and chemotherapy after surgery. While MCs and EMCs are generally considered to be non-sensitive to radiotherapy and chemotherapy, some reports have demonstrated certain efficacy.[2, 3] Case 1 in the present report experienced recurrence and metastasis after postoperative external radiotherapy, for which there was no indication for reoperation. Due to the limitation of the dose to the surrounding normal tissues, there was no indication for re-radiotherapy. Due to its small radiation radius, I-125 radioactive particles can kill tumor cells at close range inside the tumor, achieve the effect of high doses to the local tumor and low doses to the surrounding tissue, reduce the side effects of radiotherapy in surrounding normal tissue, and effectively improve the treatment gain ratio of patients. Therefore, the patient received palliative radioactive I-125 seed implantation for left parotid cancer with local treatment. Postoperative re-examination showed that the tumor in the implanted area was stable. Therefore, radioactive I-125 seed implantation can be used as an effective local treatment for patients with MC that cannot tolerate surgery, who cannot undergo resection, or with recurrence after radiotherapy. However, the patient’s submental lymph-nodes metastases outside the treatment area of the seed implantation increased significantly in volume, with multiple lung and bone metastases also increasing in size and number. Considering that after the pathological type transformation, the degree of malignancy increased and the treatment sensitivity was poor, the expected efficacy of anti-receptor is a transmembrane (EGFR) targeted therapy combined with taxane chemotherapy was also poor. Case 2 underwent systemic therapy with docetaxel, chemotherapy with paclitaxel, and anti-angiogenic with anlotinib; however, the curative effect was poor, and the tumor continued to progress with multiple metastases. The secondary biopsy revealed that the tumor had progressed to a highly malignant DC. Based on the results of genetic testing, pembrolizumab immunotherapy combined with tegafur chemotherapy showed a significant effect on the DC. However, in the later stage of disease progression, considering the drug resistance of tegafur. So pembrolizumab immunotherapy combined with gemcitabine chemotherapy was replaced with good efficacy, PR was achieved in three cycles, and has been applied for six cycles, and PR was maintained. Therefore, good effects can still be achieved by changing the new partner after drug resistance of the original scheme, and different combinations are still effective for tumor immunotherapy. To sum up, immunotherapy combined with fluorouracil or gemcitabine chemotherapy can be used to treat refractory SDCs.
4. Conclusions
MC and EMC of salivary glands are extremely rare and considered to be low-grade malignancies. Our data provide evidence that the increased degree of malignancy caused by the pathological transformation may be one of the reasons for tumor recurrence, metastasis, and resistance to treatment, resulting in poor prognosis. So, re-biopsy pathological examination is necessary after disease progression. Immunotherapy combined with chemotherapy may have good application prospects in the treatment of refractory salivary MC and EMC, however, further studies are still needed.
Conflict of Interests statement
The authors have no conflict of interest.
Conflict of funding statement
The authors have no conflict of funding.
Date access statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethic and informed consent
This cases report was approved by the institutional review board of the Hebei General Hospital. Informed consent was obtained from the patients for publication of this cases report and accompanying images.
Funding Sources
2022 Hebei Province Medical and Science Research Project Plan NO.20220071
References
- ALI J, MUNAWAR S, HAIDER R, et al. Myoepithelial Carcinoma of the Floor of the Mouth: A Rare Salivary Gland Tumor in an Unusual Location[J]. Cureus, 2020,12(12).
- WANG F, LI B, WANG Y, et al. Clinical and pathological analysis of 10 cases of salivary gland epithelial-myoepithelial carcinoma[J]. Medicine, 2020,99(41): e22671.
- THOMPSON H B, LAW M L, VASQUEZ R V, et al. Parotid Myoepithelial Carcinoma in a Pediatric Patient with Multiple Recurrences: Case Report[J]. Case Reports in Oncology, 2021,14(2): 989-997.
- MEENU B M, BHARATI H, AMIT J, et al. Salivary duct carcinoma and small cell carcinoma ex-pleomorphic adenoma: A heretofore undescribed entity and the naming conundrum: MiNEN, combined, collision, or composite tumor?[J]. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 2021,132(3).
- KARDOUNI K N, ATA G, NAZANIN M, et al. Primary acinic cell carcinoma of mandible, report of a case and literature review[J]. International Journal of Surgery Case Reports, 2021,84.
- XIA L, WANG Y, HU Y, et al. Human epithelial growth factor receptor 2 in human salivary carcinoma ex pleomorphic adenoma: A potential therapeutic target[J]. Cancer Management and Research, 2018, Volume10: 6571-6579.
- ALOS L, CARRILLO R, RAMOS J, et al. High-grade carcinoma component in epithelial-myoepithelial carcinoma of salivary glands clinicopathological, immunohistochemical and flow-cytometric study of three cases[J]. Virchows Archiv, 1999,434(4): 291-299.
- I F, A F, J S. Dedifferentiation in salivary gland carcinomas[J]. The American journal of surgical pathology, 2000,24(3).
- IKUKO O, TOSHIHIRO N, MUTSUMI M, et al. Dedifferentiated malignant myoepithelioma of the parotid gland[J]. Pathology international, 2003,53(10).
- PAROMITA R, J B M, BAYARDO P, et al. Epithelial-myoepithelial carcinoma with high grade transformation. [J]. The American journal of surgical pathology, 2010,34(9).
- AYD N S, TASKIN U, OZDAMAR K, et al. Case Study of a Parotid Gland Adenocarcinoma Dedifferentiated from Epithelial-Myoepithelial Carcinoma[J]. Case Reports in Otolaryngology, 2014,2014: 629054.
- LI B, YANG H, HONG X, et al. Epithelial-myoepithelial carcinoma with high-grade transformation of parotid gland: A case report and literature review[J]. Medicine, 2017,96(49): e8988.
- YUICHIRO H, HIROSHI H, MOTOYUKI S, et al. Salivary Duct Carcinoma of the Parotid Gland Originating from an Epithelial-Myoepithelial Carcinoma: Report of a Rare Case[J]. Head and neck pathology, 2020,14(1).
- NAOKI Y, MASAMICHI S, DAISUKE S, et al. Coexistence of salivary duct, myoepithelial and epithelial-myoepithelial carcinomas in the parotid gland: a case report and literature review[J]. Journal of Surgical Case Reports, 2021,2021(6).
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/