J Med Discov (2022); 7(2): jmd21050; DOI:10.24262/jmd.7.2.21050; Received July 22nd, 2021, Revised December 10th, 2021, Accepted April 22nd, 2021, Published July 05th, 2022.
The research progress of immune checkpoint inhibitors in gastric cancer
Sheng Chen1,Yu Peng2,Zedong Li2,Hao Liu2 and Jun Zhou*
1Department of Emergency Surgery , The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, P.R. China
* Correspondence:Dr Sheng Cheni, Department of Emergency Surgery , The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, P.R. China. Email: zhoujun15974240006@126.com
Abstract
As one of the malignant tumors with the highest incidence in the world, the morbidity and mortality of gastric cancer are high. Nearly half of the patients with gastric cancer are first diagnosed as advanced or postoperative recurrence and metastasis, which is extremely difficult to cure. Immunotherapy, as a new strategy for the treatment of gastric cancer, has developed rapidly in recent years. Based on this, the paper intends to review the latest research and application of immune checkpoint inhibitors in the treatment of advanced gastric cancer in recent years, providing reference for the clinical treatment of gastric cancer.
Keywords: gastric cancer; immune checkpoint inhibitor; immunotherapy.
Introduction
Introduction Gastric cancer is the fourth most common cancer in men after lung, prostate and colorectal cancer in the world. Besides, it is the seventh most common cancer in women after breast cancer, colorectal cancer, lung cancer, cervical cancer, thyroid cancer and uterine cancer1. According to statistics, There were about 783000 people died of gastric cancer worldwide in 2018, making it the third deadliest cancer1. The diagnosis of gastric cancer in East Asian countries is much higher than that in western countries, accounting for about half of the global diagnosis of gastric cancer due to differences in regional eating habits and medical conditions. Furthermore, patients with gastric cancer in developing countries are 5%-10% more likely to die2. As a traditional treatment for gastric cancer, chemotherapy has limited efficacy , the combination of platinum and fluorouracil is a first-line chemotherapy regimen, however, the median survival time is still less than one year3. The research progress of chemotherapy regimen is slow, there were only some changes have taken place in the mode of drug administration in the past few decades, and it is difficult to improve the prognosis of gastric cancer in a short period of time. As a new treatment for cancer, immunotherapy has been widely concerned because it provides the immune system with specificity and memory against malignant cells and achieves lasting cure with minimal toxicity. It opens up a new situation for improving the prognosis of patients with advanced gastric cancer. In the 1980s, French researchers discovered a single protein (CTLA-4) on cytotoxic T lymphocytes, and this was the beginning of immunotherapy for the treatment of cancer. Professor Allison in the United States discovered in 1996 that CTLA-4 antibodies are one of the key brakes on the immune system’s anti-tumor response, assuming that by blocking CTLA-4 molecules, the brakes will be removed and T cells will be able to attack and kill cancer cells, the hypothesis was then tested in mice4. Professor Allison was awarded the 2018 Nobel Prize in Physiology or Medicine for his work. By 2011, ipilimumab, a CTLA-4 checkpoint inhibitor, had been approved by the FDA for the treatment of metastatic melanoma5. In 2013, the journal Science declared cancer immunotherapy the “Breakthrough of the Year”6. Now cancer immunotherapy is progressing rapidly, a number of new drugs(nivolumab、Pembrolizumab、Avelumab、 Atezolizumab、 Durvalumab 、 Cemiplimab) 7-12have been approved to be put on the market(Table 1). Immunotherapy also has made great progress in advanced gastric cancer, and the therapeutic effect of immune checkpoint inhibitors has been confirmed. This paper reviews the progress of immunotherapy in gastric cancer in order to provide new ideas for the treatment of gastric cancer.
Table 1 The dates immune checkpoint inhibitors for cancer were first approved by FDA
| Drugs | Target | Date approved | Idcations |
| Ipilimumab | CTLA-4 | 3/25/2011 | metastatic melanoma |
| Nivolumab | PD-1 | 12/22/2014 | Unresectable or metastatic melanoma cancer that has progressed following treatment with ipilimumab, or a BRAF inhibitor in BRAF mutation-positive patients |
| Pembrolizumab | PD-1 | 9/4/2014 | metastatic melanoma patients who are refractory to CTLA-4 therapy and BRAF inhibitor if they have BRAF mutation |
| Avelumab | PD-L1 | 11/18/2015 | Metastatic Merkel cell carcinoma in patients ≥ 12 years |
| Atezolizumab | PD-L1 | 5/18/2016 | Locally advanced or metastatic urothelial carcinoma that has progressed during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy |
| Durvalumab | PD-L1 | 5/1/2017 | Locally advanced or metastatic urothelial carcinoma patients with disease progression during or following platinum-containing chemotherapy, or whose disease has progressed within 12 months of receiving platinum-containing chemotherapy neoadjuvant or adjuvant |
| Cemiplimab | PD-1 | 9/28/2018 | Metastatic or locally advanced cutaneous squamous cell carcinoma who are not the candidate for curative surgery or radiation |
Anti-tumor mechanism of immune checkpoint inhibitor
Immune cells can recognize and eliminate mutant cells under normal circumstances. This immune surveillance can prevent the development of cancer. However, cancer cells usually proliferate rapidly by evading immune surveillance13. Classical T cell activation requires three signals: (1) The interaction between T cell receptor (TCR) and major histocompatibility complex (MHC), (2) Costimulatory signal, that is, the interaction between CD28 molecule expressed on T lymphocytes and CD80/CD86 expressed on antigen presenting cell (APC), and (3) IL-2/IL-2 receptor signal pathway. These three signals lead to lymphocyte cycle progression, survival and differentiation13. Immune checkpoint molecules are inhibitory receptors on immune cells, which trigger immunosuppressive signal transduction pathways. They are important for maintaining self-tolerance and regulating the duration and amplitude of physiological immune response in surrounding tissues to minimize collateral tissue damage. Cancer cells promote mutual inhibition between immune cells through certain immune checkpoint pathways to escape immune control15,16. On the other hand, immune checkpoint inhibitors promote immune activation by removing the inhibitory effect of cancer immune system, hinder negative immunoregulatory receptors and signals, enhance tumor immunity and make it play a better anti-tumor effect. Several studies have observed that in a variety of cancer patients receiving these molecular treatments. The clinical benefits of patients are associated with increased proliferation of CD8+ memory T cells17-19, enhanced T cell toxicity and antigen-specific T cell production of pro-inflammatory cytokines in tumors20. At present, the immune checkpoints of cytotoxic T lymphocyte associated antigen-4 (CTLA-4) and programmed death factor-1 (PD-1) have been deeply studied, which has become an important target for tumor immunotherapy.
Anti-CTLA-4
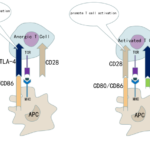
CTLA-4 is a member of the immunoglobulin superfamily and a co-inhibitory receptor. TCR/MHC signal induces activated T cells to express CTLA-4, which competes with costimulatory receptor CD28 to bind B7 molecule (CD80/CD86)21. In addition, the binding affinity of binding CD80/CD86 of CTLA-4 is higher than that of CD28. CTLA-4 is mainly expressed on T cells, and also expressed in other immune cells, including B lymphocytes and fibroblasts22,23. It plays a powerful role in the negative regulation of T cell activation24. Blocking CTLA-4 can enhance the CD4+ activity of effector T cells. Therefore, monoclonal antibodies against CTLA-4 can enhance anti-tumor immunity.(Fig. 1)
Figure1. Negative regulation of CTLA-4 on T cells
Temelimumab monoclonal antibody is a humanized monoclonal antibody against CTLA-4. The results of Temelimumab monoclonal antibody in the treatment of advanced gastric cancer and gastroesophageal junction adenocarcinoma showed that 1 patient was in partial remission in the II phase clinical study of 18 patients in 201025. Although Temelimumab showed good anti-tumor activity in malignant melanoma, renal cell carcinoma, mesothelioma, non-small cell lung cancer and other tumors, there was no other related data in gastric cancer.
Ipilimumab is another fully humanised monoclonal antibody against CTLA-4 (IgG1). Ipilimumab promotes anti-tumor response through T cell activation and the infiltration of T cell into tumor26,27. Ipilimumab demonstrated its clinical efficacy against metastatic melanoma in two phase 3 clinical trials28,29,and it was first approved by FDA in 2011 for the treatment of advanced melanoma30. For some other tumor types Ipilimumab also shows significant OS benefits31-34. A phase II clinical trial35 showed that Ipilimumab monotherapy did not improve immune-related progression-free survival (irPFS) compared with the best supportive therapy. However, Ipilimumab monotherapy had a median overall survival(mOS)of nearly 1 year and showed an excellent safety profile, which supports the study of Ipilimumab combined with other therapies in the treatment of advanced gastric cancer. The cohort study CheckMate03236 showed that the combination of Ipilimumab and Nivolumab had a better therapeutic effect than single Nivolumab. The mOS and objective remission rate(ORR)for advanced gastric cancer were 6.9 months and 24%, respectively, showing a good response rate and a long response time. In the meanwhile, subgroup analysis shows that the effective rate of combined immunotherapy in people with microsatellite instability-high(MSI-H) can be as high as 50%, and had a favorable safety profile. The third phase study to evaluate Nivolumab or Nivolumab plus Ipilimumab in the treatment of gastric cancer is under way.
Anti-PD-1/PD-L1
As a small immune checkpoint molecule and a co-inhibitory molecule, PD-1 is widely expressed on T cells, especially on antigen-specific CD8+T cells in tumors37, as well as on B cells, thymocytes, natural killer cells and myeloid dendritic cells (DC). PD-1 binds to its natural ligand PD-L1 (B7-H1) or PD-L2 (B7-DC) to provide a signal for the termination of immune system activity and protect normal tissue in an inflammatory environment. Tumor cells use this mechanism to lead to immune escape and tumor progression. PD-1/PD-L1 interaction induces T lymphocyte depletion, apoptosis, and anergy38-42. As a consequence, anti-PD-1 antibodies can promote the activation of T cells to kill tumor cells43.
Pembrolizumab is a highly specific monoclonal antibody against PD-1. For the first time, the study KEYNOTE-01244 evaluated the safety and efficacy of pembrolizumab in the treatment of advanced gastric cancer. A total of 39 PD- L1 positive patients were treated with Pembrolizumab every two weeks. The results of follow-up to 8.8 months showed that 53% of the patients had tumor regression, 22% achieved partial remission, and the median duration of remission was 40 weeks, which is a prelude to immunotherapy for gastric cancer. The results of phase II KEYNOTE-059 trial45 showed that the objective remission rate (ORR) of pembrolizumab group was 12%, the median progression-free survival(PFS) was 2 months, and the median OS was 5.5 months, indicating that PD-L1 positive was more likely to benefit from Pembrolizumab. Based on this study, in September 2017, FDA approved Pembrolizumab as a third-line treatment for advanced gastric cancer with comprehensive positive score (CPS) ≥ 1 and PD-L1 positive. According to the research of Le et al46, the ORR of Pembrolizumab in the treatment of high-MSI solid tumors reached 53%.As a consequence, FDA approved Pembrolizumab as the second-line treatment for high-MSI/deficient mismatch repair(dMMR) solid tumors in 2017. The phase III study KEYNOTE-06147 was first reported at the ASCO conference in 2018. This study compared the efficacy of Pembrolizumab and paclitaxel in the second-line treatment of gastric cancer / gastroesophageal junction cancer with CPS ≥ 1. The results showed that Pembrolizumab group was better than paclitaxel group in mOS (9.1 months vs 8.3 months, P= 0421) and mPFS (1.5 months vs 4.1 months) had no significant prolongation, but Pembrolizumab had better safety profiles. KEYNOTE-062 is a phase III clinical trial48 to explore the value of immunotherapy in the first line of gastric cancer.A total of 763 patients were randomly assigned to Pembrolizumab monotherapy group(n = 256), Pembrolizumab with cisplatin plus fluorouracil or capecitabine group(n = 257), placebo with cisplatin plus fluorouracil or capecitabine group(n = 250). The results showed that in the patients with CPS ≥ 1, the median OS of the Pembrolizumab monotherapy group compared with the chemotherapy group was 10.6 months vs. 11.1 months (P=0.162), and the study reached the end point of non-inferiority. Among the patients with CPS ≥ 10, the Pembrolizumab monotherapy group was significantly better than the chemotherapy group, the median OS of the two groups was 17.4 months vs. 10.8 months respectively. Safety comparison shows that Pembrolizumab is more tolerable.This is the only clinical trial in which PD-1 monoclonal antibody achieves non-inferiority compared with standard chemotherapy. However, whether in patients with CPS ≥ 1 or CPS ≥ 10, there was no significant improvement in OS in the immunotherapy group. It is suggested that immunocombination chemotherapy for first-line treatment of advanced gastric cancer needs further study. Nevertheless, patients with advanced gastric cancer with CPS ≥ 10 may benefit from single pembrolizumab therapy.
Nivolumab is a monoclonal antibody targeting PD-1.The study ATTRACTION-249 conducted in Japan and South Korea in 2017 showed that there was a significant difference in mOS between the Nivolumab group and the placebo group (5.26 months vs4.14 months, P < 0.0001). Additionally, the level of PD-L1 expression did not affect the benefits of the patients. Based on the study, In September 2017, Japan approved nivolumab for third-line treatment of advanced after chemotherapy, unresectable or recurrent gastric cancer. Nivolumab showed a better overall survival rate than placebo in patients with advanced gastric cancer or gastroesophageal junction cancer in Asia. CheckMate-03236 evaluated the safety and efficacy of nivolumab and nivolumab plus ipilimumab in the treatment of gastric cancer. After strict pre-selection, all the selected patients were Western patients with metastasis of gastric cancer after the failure of second-line chemotherapy As a result, it showed that nivolumab and nivolumab plus ipilimumab have clinical significance and lasting anti-tumor activity in western gastric cancer patients, and the safety can be controlled. This suggests that despite the morphological and molecular heterogeneity of gastric cancer, immune checkpoint inhibitors provide consistent therapeutic benefits in Asian and Western patients. Phase II trial ATTRACTION-450 showed that the ORR of nivolumab plus SOX regimen (S-1 + oxaliplatin) and nivolumab combined with XELOX regimen (capecitabine + oxaliplatin) as first-line treatment for advanced HER-2-negative gastric cancer was 57.1% and 76.5%, respectively. In addition, the proportion of patients who interrupted treatment due to related adverse reactions was 10%, and there were no treatment-related deaths and new adverse reactions. It is suggested that the regimen of Nivolumab combined with chemotherapy is safe and available. Besides, nivolumab is expected to be the first immunocheckpoint inhibitor approved for first-line treatment of advanced HER-2-negative gastric cancer.
Avelumab is a highly specific monoclonal antibody against PD-L1. Phase Ⅲ trial JAVELIN Gastric 30051 revealed that mPFS (1. 4 months vs 2. 7 months, P > 0. 99) and mOS (4. 6 months, vs 5. 0 months, P = 0. 81) between Avelumab group and chemotherapy group had no significant difference, but there were fewer bad events in the Avelumab group. It suggested that Avelumab was more safe, but it could not be used as a third-line treatment for advanced gastric cancer. Phase III trial JAVELIN Gastric 10052 was conducted to study the efficacy of Avelumab versus continuous chemotherapy in the treatment of gastric cancer after first-line induction chemotherapy. 499 patients were randomly assigned to Avelumab group (n=249) and continuous chemotherapy group (n= 250). The results indicate that the median total survival time of Avelumab group was 10.4 months (95% CI, 9.1 to 12.0 months). The median survival time of continuous chemotherapy group was 10.9 months (95% CI, 9.6 to 12.4 months). In PD-L1 (+) patients, the median total survival time was 14.9 months in Avelumab group and 11.6 months in continuous chemotherapy group. Whether in all randomized or PD-L1 (+) GC/GEJC patients, Avelumab maintenance therapy did not show a superior role of OS. In consequence, the therapeutic effect of PD-L1 and its antibody in advanced gastric cancer needs to be further explored.
Conclusion
Immunotherapy has successfully ushered in a new era of cancer treatment. There is no doubt that although immunotherapy is developing rapidly, there are still many uncertainties, such as the lack of biomarkers for accurate prediction of curative effect. In the field of gastric cancer , the only clear biomarkers are MMR status and PD-L1 expression, and the predictive role of other biomarkers (such as total mutation load, etc.) is not clear. It is still the direction of our efforts to transform the results of laboratory research into clinical trial research as soon as possible. There are still many clinical trials on immunotherapy for gastric cancer, not only the efficacy of immune monotherapy, but also the trials of immunotherapy combined with other therapies are also being carried out. More and more evidence shows that chemotherapy plus immunotherapy can enhance the anti-tumor effect, and the combination therapy shows a good remission rate and survival benefit, which is also an important strategy to overcome drug resistance. Generally speaking, to combine with chemotherapy is a major direction of immunotherapy in the future.
Conflict of interest
None
Acknowledgments
None
References
- Bray, F.et al., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68 394 (2018).
- Guggenheim, D. E. & Shah, M. A., Gastric cancer epidemiology and risk factors. J SURG ONCOL107 230 (2013).
- Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F., Gastric cancer. LANCET396 635 (2020).
- Leach, D. R., Krummel, M. F. & Allison, J. P., Enhancement of antitumor immunity by CTLA-4 blockade. SCIENCE271 1734 (1996).
- Hodi, F. S.et al., Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363 711 (2010).
- Couzin-Frankel, J., Breakthrough of the year 2013. Cancer immunotherapy. SCIENCE342 1432 (2013).
- Weber, J. S.et al., Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. LANCET ONCOL 16 375 (2015).
- Chen, R.et al., Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J CLIN ONCOL 35 2125 (2017).
- Kaufman, H. L.et al., Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J IMMUNOTHER CANCER 6 7 (2018).
- Balar, A. V.et al., Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. LANCET 389 67 (2017).
- Powles, T.et al., Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA ONCOL 3 e172411 (2017).
- Migden, M. R.et al., PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 379 341 (2018).
- de Visser, K. E., Eichten, A. & Coussens, L. M., Paradoxical roles of the immune system during cancer development. (2006).
- Coutzac, C., Pernot, S., Chaput, N. & Zaanan, A., Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol133 25 (2019).
- Pardoll, D. M., The blockade of immune checkpoints in cancer immunotherapy. NAT REV CANCER12 252 (2012).
- Baumeister, S. H., Freeman, G. J., Dranoff, G. & Sharpe, A. H., Coinhibitory Pathways in Immunotherapy for Cancer. ANNU REV IMMUNOL34 539 (2016).
- Iwai, Y., Terawaki, S. & Honjo, T., PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. INT IMMUNOL17 133 (2005).
- Ribas, A.et al., PD-1 Blockade Expands Intratumoral Memory T Cells. CANCER IMMUNOL RES 4 194 (2016).
- Wong, R. M.et al., Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. INT IMMUNOL 19 1223 (2007).
- Simon, S.et al., PD-1 expression conditions T cell avidity within an antigen-specific repertoire. ONCOIMMUNOLOGY 5 e1104448 (2016).
- Rowshanravan, B., Halliday, N. & Sansom, D. M., CTLA-4: a moving target in immunotherapy. BLOOD131 58 (2018).
- Quandt, D., Hoff, H., Rudolph, M., Fillatreau, S. & Brunner-Weinzierl, M. C., A new role of CTLA-4 on B cells in thymus-dependent immune responses in vivo. J IMMUNOL179 7316 (2007).
- Allison, J. P. & Krummel, M. F., The Yin and Yang of T cell costimulation. SCIENCE270 932 (1995).
- Grierson, P., Lim, K. H. & Amin, M., Immunotherapy in gastrointestinal cancers. J Gastrointest Oncol8 474 (2017).
- Ralph, C.et al., Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. CLIN CANCER RES 16 1662 (2010).
- Walunas, T. L.et al., CTLA-4 can function as a negative regulator of T cell activation. IMMUNITY 1 405 (1994).
- Grosso, J. F. & Jure-Kunkel, M. N., CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun13 5 (2013).
- Hodi, F. S.et al., Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363 711 (2010).
- Robert, C.et al., Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364 2517 (2011).
- Buchbinder, E. I. & McDermott, D. F., Cytotoxic T-lymphocyte antigen-4 blockade in melanoma. CLIN THER37 755 (2015).
- Lynch, T. J.et al., Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J CLIN ONCOL 30 2046 (2012).
- Reck, M.et al., Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. ANN ONCOL 24 75 (2013).
- Yang, J. C.et al., Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J IMMUNOTHER 30 825 (2007).
- Slovin, S. F.et al., Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. ANN ONCOL 24 1813 (2013).
- Bang, Y. J.et al., Efficacy of Sequential Ipilimumab Monotherapy versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. CLIN CANCER RES 23 5671 (2017).
- Janjigian, Y. Y.et al., CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J CLIN ONCOL 36 2836 (2018).
- Ahmadzadeh, M.et al., Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. BLOOD 114 1537 (2009).
- Barber, D. L.et al., Restoring function in exhausted CD8 T cells during chronic viral infection. NATURE 439 682 (2006).
- Deng, R.et al., B7H1/CD80 interaction augments PD-1-dependent T cell apoptosis and ameliorates graft-versus-host disease. J IMMUNOL 194 560 (2015).
- Goldberg, M. V.et al., Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. BLOOD 110 186 (2007).
- Martin-Orozco, N., Wang, Y. H., Yagita, H. & Dong, C., Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J IMMUNOL177 8291 (2006).
- Probst, H. C., McCoy, K., Okazaki, T., Honjo, T. & van den Broek, M., Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. NAT IMMUNOL6 280 (2005).
- Grierson, P., Lim, K. H. & Amin, M., Immunotherapy in gastrointestinal cancers. J Gastrointest Oncol8 474 (2017).
- Muro, K.et al., Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. LANCET ONCOL 17 717 (2016).
- Fuchs, C. S.et al., Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA ONCOL 4 e180013 (2018).
- Le DTet al., Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. SCIENCE 357 409 (2017).
- Shitara, K.et al., Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. LANCET 392 123 (2018).
- Shitara, K.et al., Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA ONCOL 6 1571 (2020).
- Kang, Y. K.et al., Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. LANCET 390 2461 (2017).
- Boku, N.et al., Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). ANN ONCOL 30 250 (2019).
- Bang, Y. J.et al., Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. ANN ONCOL 29 2052 (2018).
- Moehler, M.et al., Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J CLIN ONCOL O2000892 (2020).
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/