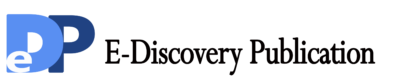J Med Discov (2020); 5(3):jmd20040; DOI:10.24262/jmd.5.3.20040; Received June 18th, 2020, Revised July 10th, 2020, Accepted July 27th, 2020, Published August 16th, 2020.
Waste Herbal and Black Tea as a Novel Adsorbent for Detoxification of Pharmaceutical Effluent
Sepideh Arabian1, Parisa Ziarati 1,*, Barbara Sawicka2
1Nutrition & Food Sciences Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran-Iran.
2Department of Plant Production Technology and Commodities Sciences, Faculty of Agrobioengineering, University of Life Sciences in Lublin, Poland.
* Correspondence: Parisa Ziarati,Nutrition and Food Sciences Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran-Iran, No 99, Yakhchal, Gholhak, Dr. Shariati, Tehran -Iran. E-mail: ziarati.p@iaups.ac.ir
Abstract
The metal bio-adsorption is the removal of metal ions by inactive, nonliving biomass due to highly attractive forces present between them. The use of Agro-industrial wastes as raw materials to reduce cost production and pollution. The method designed based on Agricultural Waste as a compost and bio-adsorbent for detoxification of Heavy Metals and Toxic contaminations from soils. In current study the potential of conventional beverage black tea residue which is thrown away in many houses and produced by an infusion of leaves of the evergreen shrub Camellia sinensis, a member of the aceae family and also one of popular herbal tea, sage leaves(Salvia officinalis) which mostly consumed by people due to medicinal properties. Some factors and various parameters like initial concentration, pH, contact time, temperature, agitation speed and bio-adsorbent dose were studied. One way analysis of variance (ANOVA) was used for data analysis to measure the variations of metal concentrations using SPSS 22.0 software. Effect of various pH; temperature; dose of S. officinalis in comparison of black tea residue after infusion of 10 minutes separated and studied on remediation and detoxification of contaminated wastewater effluent from pharmaceutical laboratories effluents in different contact times and initial concentrations, particle size and agitation speed were studied . The samples were analyzed by standardized international protocols. The best results of cadmium and cobalt removal obtained by S. officinalis (0.5%) while black tea residues (0.5%) after 2 weeks revealed the best results. Further increase in contact time more than 48 hours did show significant increase in bio-adsorption potential and agitation factor showed a significant role (p < 0.001) in adsorption process. The results of current study revealed that using S. officinalis and black tea residue have the high potential of removing and decreasing cadmium, cobalt and nickel concentrations significantly (p< 0.03) from wastewater but the black tea bio-mass has significant more potential (p < 0.05) in removal of Cadmium and Cobalt.
Keywords: Detoxification, Pharmaceutical Effluent, Bio-mass, Sage, Salvia officinalis, black tea residue.
Introduction
According to previous studies in Iran, large scale industrialization is mainly responsible for severe and high levels of heavy metal concentrations in the farmlands and regional environments. This threatens not only to the physical well-being of individuals but endangers “human” existence physically, sociologically and psychologically [1-12].The heavy metals toxicity lead to biogeochemical impacts on neuronal disease and synergist concomitant violence in society and general poor health , which is in remarkable and critical state for elderly people as well as young generation. The accumulation of toxic and heavy metals is the natural geological and geogenic and environments input of metal (oids) [1, 13-15]. Metals in pathology are an ever increasing issue for health. Geological releases of cadmium to soil, surface and ground waters is diverse and even includes aerosols. Hydrothermal degassing emissions through fumarolic, diatreme and larger volcanic vents to atmosphere add significant Cd to the food chain [1]. What is urgent needed in many developing countries and even developed ones is apply geochemical and mineralogical techniques to pathology and the study of internal metabolic pathways and cell signaling. Both essential and harmful links between Earth materials and human health should be more fully concerned particularly excesses or deficiencies of elements, ions and key micronutrients such as iodine, selenium, iron, arsenic, and radon. Although as trace elements some heavy metals are required in to maintain the human body metabolism. Unfortunately poisoning naturally occurs at higher contents of such heavy metals. Heavy metals are dangerous and toxic in nature because they tend to bio-accumulation living cells where the concentration of a specific chemical or a heavy metal increases the non-degradable toxic in nature [1, 16-23]. In current study we focused on some heavy metals such as Cadmium, Cobalt and Nickel due to the abundance of them in pharmaceutical effluents from educational and research laboratories in Pharmaceutical faculty in Tehran Medical Sciences, Islamic Azad University and the high risk factors of these elements and on the other hand the project of programs of managing waste materials. The programs of managing waste materials in developing countries are often unsatisfactory and the unreasonable disposal of waste is a major issue worldwide [24-26]. Likewise, conventional methods of remediation of water and soil are particularly expensive and energy consuming and the elevated costs involved in removal of toxic substances from contaminated water and soils prevent repudiation from being carried out; especially in areas of little money-making value [27-31]. In current study wastewater generated from the educational and research pharmaceutical laboratories varies drastically in the pH ranging from acidic to alkaline For example, the pH of an alkaline waste stream from a synthetic organic pharmaceutical plant ranges from 9 to 10, whereas a pH of 0.8 has been reported for acidic waste streams7, 8 and from Toxicology laboratory and also Nutrition and Food Industries Research center 2-4.Nevertheless, almost all types of waste streams produced from the pharmaceutical research laboratories are either alkaline or acidic.The use of bio-adsorbents for removal of toxic heavy metals from waste offers a relatively low-cost method with potential for metal recovery [30-32].
Adsorption has come out as effective, low-cost, environmentally and ecofriendly treatment technique for treating contaminated soils and wastewaters [14-32]. The removal or decreasing of toxic and heavy metals by using low cost agricultural or food waste adsorbent found to be more supportive in prolonged designation as there are considerable components existing locally and profusely such as natural materials, agricultural wastes or Food industrial by-products which can be applied as environmentally and economic adsorbents[33]. Agricultural waste as a bio-adsorbent for detoxification of Heavy Metals and Toxic contaminations from wastewater could be one of the best low-cost and effective methods nowadays. The technique of adsorption is defined as a process where the atoms, ions or molecules of dissolved solids from liquid grips on the surface of solid; i.e. it is a process of mass transfer in which the dissolved solid from liquid gets deposited on the surface of solid because of physical / chemical interaction.
In this study we focused on two tea residues (black and herbal Sage tea). Salvia officinalis L. (Sage) is a perennial round shrub in the family of Labiatae/ Lamiaceae. Plants of this genus grow all over the world and the specie of S. officinalis is native to Middle East and Mediterranean areas. Today’s, it has been naturalized throughout the world particularly in Europe and North America [34-36]. Because of its flavoring and seasoning properties, this plant has been widely used in preparation of many foods. In folk medicine of Asia and Latin America, it has been used for the treatment of different kinds of disorders including seizure, ulcers, gout, rheumatism, inflammation, dizziness, tremor, paralysis, diarrhea, and hyperglycemia [37 -38]. Particularly, it is due to the presence of certain functional groups, such as amine, carboxyl, hydroxyl, phosphate, sulfhydryl etc., on the cell wall of the biomass. In current study, the main goal is finding suitable green method for some heavy metals such as Cadmium, Nickel and Cobalt removal from pharmaceutical effluent laboratories by bio-mass of tea residues as a cheap citrus waste in a batch system by considering the effects of various parameters like initial concentration, pH, contact time, agitation speed and bio-adsorbent dose.
Materials and Methods
Pharmaceutical Effluent
Effluents from 5 research laboratories in pharmaceutical Faculty, Tehran Medical Sciences, including, Nutrition and Food Sciences Research Center (Effluent 1&2), Educational and research Toxicology labs (Effluent 3&4), Analytical chemistry (Effluent 5) were used in current study. Effluent 1 and 2 were from the same laboratory but were collected on separate occasions with a 2 week time interval. Although Effluent 1 and Effluent 2 come from the same wastewater treatment plant, they were examined and considered as 2 different effluents due to the variability of their characteristics. The characteristic is attributed to the significant experiments which occurred following the first sampling event. After collection, the wastewater effluent was instantly transported to the main research laboratory for analysis. Physico-chemical parameters such as Total Solids, Total hardness, pH, Electrical Conductivity, Total Dissolved Solids, Chloride, Sulphate, Dissolved oxygen, Nickel, Cadmium, Lead, Zinc, Copper, Chrome, Manganese, Iron and Potassium were analyzed as per the standard methods and results showed in table 1.
The initial concentration of heavy metals/metalloid such as Nickel, Zinc, Copper, Cobalt and Lead in the untreated effluents and treated by herbal tea residue from Salvia officinalis L. (Sage), and also black tea residue were analyzed. After especial times: 48, 72 hours and 1 and 2 weeks (by/without stirring) final concentration of heavy metals in effluent samples were analyzed using Atomic Absorption Spectroscopy. The samples were analyzed by an Atomic Absorption Spectrophotometer Model AA-6200 (Shimadzu, Japan) using an air-acetylene flame for heavy metals and using six standard solutions for each metal. All required precautions were taken to avoid any possible contamination of the sample as per the AOAC guidelines [31-36].The efficiency of conventional and herbal tea residues in bio-adsorbing Cobalt, Cadmium and Nickel was investigated.
Material preparation
Black tea and herbal tea residues were collected from recognized centers and coffee shops and even from household thrown away in Tehran in July 2018 – March 2020.
Thereafter, the residues samples were subjected to a crusher to reduce particle sizes and then dried again for 4 hours at 85°C. The powder of residues used during the Bio-adsorption study was at natural state with no chemical or thermal treatment.
Chemicals
All the chemicals used in current project were of analytical grade and actual Pharmaceutical effluent and wastewater samples were collected in 5 groups of 5L bottles from Chemical Laboratories After collection, the wastewater effluent was instantly transported to the main research laboratory for analysis. Physico-chemical parameters such as pH, Dissolved oxygen, Total Solids, Total hardness, Electrical Conductivity, Total Dissolved Solids, Chloride, Sulphate, Calcium, Sodium, Cadmium, Lead, Nickel, Zinc, Copper, Chrome, Manganese ,Iron and Potassium were analyzed as per the standard methods [ 25-26, 39 ].
The synthetic Sigma-Aldrich stock solution of Cd+2, Ni +2 and Co+3 (1000mg/L) were used for making standard solutions. The desired concentrations of six standard solutions for the metals were prepared by successive dilutions of the stock solution.
Adsorbent particle size
The crusher sieve was used for the reduction of particle size of the dried black and herbal tea residues samples and for particle size distribution, respectively. The sieves were mechanically vibrated for 10 minutes which was sufficient for separation to take place. The particle size range used in this study was 0.15 mm to 5 mm.
Statistical Analysis
The values reported in current study are means of three values. Data were tested at different significant levels using student t-test to measure the variations between the contaminations in wastewater and the dose of bio-adsorbent and contact time parameters before and after treated by tea residue bio-adsorbents in 2 forms of black and herbal (sage) tea. One way analysis of variance (ANOVA) was used for data analysis to measure the variations of metal concentrations using SPSS 22.0 software (SPSS Inc, IBM, Chicago, IL).
Results
The samples were analyzed by wet digestion method and standardized international protocols [39] were followed for the preparation of material and analysis of Cadmium, Nickel and Cobalt contents and analyzed by Atomic Absorption Spectrophotometer in Research Laboratory of Nutrition and Food Sciences Research Center, in Pharmaceutical faculty, Tehran Medical Sciences. The data obtained from chemical analyses, mean values were calculated and are given in the table 1. The results of characteristics of pharmaceutical effluent from all samples gathering from all laboratories in November 2019 were analyzed are showed in table 1.
Table 1- Characteristics of studied Pharmaceutical Effluent from Pharmacy Faculty
| Parameters | Concentration Range | Average |
| pH | 0.18 – 6.8 | 4.1 |
| BOD5 (mg/L): Biological oxygen demand | 2011–3241 | 2600 |
| COD (mg/L): chemical oxygen demand | 1200 – 7200 |
2448
|
| TSS (mg/L): total suspended solids | 30 – 65 |
43
|
| Total alkalinity as CaCO3(mg/L) | 70 – 1540 |
780
|
| Lead (mg/L) | 1.112 – 21.303 | 8.561 |
| Tin (mg/L) | 0.9 – 11.4 | 6.5 |
| Cadmium (mg/L) | 3.581 – 10.041 | 6.54 |
| Mercury (mg/L) | 0.11 – 0.38 |
0.19
|
| Nickel (mg/L) | 110.19-376.81 |
323.12
|
| Cobalt (mg/L) | 51.651-105.005 | 87.156 |
| Chromium (mg/L) | 5.89 – 10.54 | 6.09 |
| Chloride (mg/L) | 500 – 1360 | 894 |
| Sulfide (mg/L) | 2-18 | 8 |
| Nitrate (mg/L) | 350-1980 |
1100
|
As compared to BOD, COD was very high which is normal for pharmaceutical effluent of such educational and research laboratories especially for toxicology studies. The minimum and maximum values ranged between 2011–2600 and mean of 2573 mg/L and the average values ranged between 1200 – 7200and mean of 2448 mg/L for the studied effluent.
The effect of the concentrations of adsorbent on the removal of Cd +2 ions by Black tea residue is depicted in figure 1, for varied adsorbent doses of 0.1%, 0.2%,0.3%, 0.4%, 0.5%, w/v .The results revealed that the potential of 0.5% Bio-adsorbent after 2 weeks by stirring the wastewater solution can decrease the 6.54 mg/Lit to the 0.767 mg/Lit Cadmium content which means black tea bio-mass removed significantly 88.27% by waste low-cost material ( p < 0.002 ). In less contact time of 72 hours and only 5% bio-mass the results showed that decreasing of Cd was significantly effective (p < 0.03).
Figure1. Effect of contact time on the removal of cadmium (initial Cd concentration=6.543 mg/Lit,bio-adsorbent dose=0.1% ,0.2%,0.3%,0.4% and 0.5% W/V black tea residues in pharmaceutical Effluent,temperature=25 ± 2 ºC,agitation speed= 200 rpm), pH = 4.1.
Results in figure 1 showed significant difference in Cadmium up -taking by bio-adsorbent after 48 and 72 hours and also 7 days (1 week) without stirring. The data showed that accomplishing of agitation speed=200 rpm during 1 week and 14 days (2 weeks) and the same experience but without stirred have significant differentiate (p<0.05) , but the potential of taking up Cadmium was increased significantly between 2 week with agitation speed by 2 weeks in statistic state (p<0.002 ).Moreover, time factor of putting adsorbent in contaminated effluent wastewater by cadmium in the study showed significant effect (p<0.05) and positive correlation with contents of Cd (r = +90 to r = +94), in the contaminated wastewater and black tea residue in one week contact respectively. The amounts of Cadmium adsorbed increased significantly with increase contact time (p<0.001).The other factor of stirring solution also showed significant agent to decreasing and removal of heavy metal from wastewater (p<0.002). The data revealed that utilizing 0.5 % bio-adsorbent of Black tea residue as a household waste has the great potential of removing 88% of cadmium at room temperature,which introduce a green environmentally method for detoxification of wastewaters.
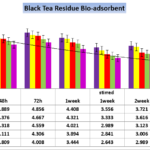
Figure 2. Effect of contact time on the removal of Cobalt (initial Co concentration=87.165 bio-adsorbent dose=0.1%, 0.2%, 0.3%,0.4% and 0.5% W/V black tea residues in pharmaceutical Effluent , temperature=25 ± 2 ºC, agitation speed= 200 rpm), pH = 4.1.
On the contrary of all data and results of cadmium removal and the stirring agent as the most important factor in figure 2 results showed significant difference in Cobalt up -taking by bio-adsorbent after 72 hours and 7 days and also 7 days (1 week) with stirring. The data showed that accomplishing of agitation speed=200 rpm during 1 week and 14 days (2 weeks) without stirred have no significant differentiate (p ≥ 0.05),but the potential of taking up Cobalt was increased significantly between 2 week with agitation speed by 2 weeks in statistic state (p<0.001 ) and Cobalt content in untreated sate 87.165 mg/Lit after 2 weeks contact time decreased to 30.188 mg/Lit which prove that biomass of adsorbent can reduce 65.37% of heavy metal.Moreover, time factor of putting adsorbent in contaminated effluent wastewater consist of high concentration of Cobalt in the study showed significant effect (p<0.032 and positive correlation with contents of Cd (r = +889 to r = +92), in the contaminated wastewater and Black tea residue in one week contact respectively. The amounts of Cobalt adsorbed increased significantly with increase contact time (p<0.003) in agitated conditions.The other factor of boosting bio-adsorbent also showed significant factor to decreasing and removal of heavy metal from wastewater (p<0.001) and best results achieved in presence of 0.5% residue and more concentrations of black tea had not significant effect on removing Cobalt removal. Therefore the higher content of bio-mass would not reveal in this paper.
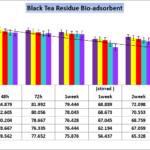
Figure 3. Effect of contact time on the removal of Nickel (initial Ni concentration=323.12 bio-adsorbent dose=0.1%, 0.2%, 0.3%,0.4% and 0.5% W/V black tea residues in pharmaceutical Effluent, temperature=25 ± 2 ºC, agitation speed= 200 rpm), pH = 4.1.
The results shown in figure 3,regarding Nickel removal from contaminated wastewater revealed that even utilizing very low concentration of bio-mass waste and residue of black tea has the remarkable and significant potential (p<0.001) in current green and eco-friendly project. After 2 weeks contact time there is no significant difference among percentage of Black tea wastes and in all concentrations the decreasing Nickel significantly removed most part of heavy metals (84.5%). By 0.5% bio-adsorbent and after 2 weeks contact time and agitation of 200 rpm,the initial concentration of Nickel declined to 50.61 mg/Lit;which means 84.34% efficacy and significant detoxification of pharmaceutical effluents (<0.001).
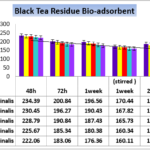
In the second part of current study we have caparisoned the potential of Sage and herbal tea by black tea residues. As it is shown in figure 4, Cadmium removal in presence of the same percentage of biomass on the same pharmaceutical effluent was investigated.
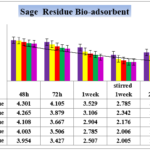
Figure 4. Effect of contact time on the removal of cadmium (initial Cd concentration=6.543 bio-adsorbent dose=0.1% ,0.2%,0.3%,0.4% and 0.5% W/V Sage Herbal tea residues in pharmaceutical Effluent,temperature=25 ± 2 ºC, agitation speed= 200 rpm), pH = 4.1.
Sage herbal tea residue and results revealed the potentially increased from 70.93. % by 0.5% biomass which means that treated by chelation agents with the increase of the potential of bio-absorbent, removal ability of heavy metals by sage leaves tea residue without treatment by any chemical ingredients and in different varied from 40.21% to 70.93% depending on different concentrations of bio-adsorbents and also agitation speed and the best results obtain in 0.5% sage leave tea residue temperature=25 ± 1 ºC, agitation speed= 200 rpm in pH = 4.1.
Results in figure 4 in presence of 1% up to 5% biomass wastes showed significant difference in Cadmium up -taking by bio-adsorbent after 48 and 72 hours and also 7 days/14 days [1 and 2 week(s) ] without stirring and also stirring. The data indicated that accomplishing of agitation speed= 200 rpm during 1 week and 14 days (2 weeks) without stirred have significant differentiate (p < 0.05) just like black tea bio-mass concentrations of adsorbents in this study, but the potential of taking up Cadmium was increased significantly between 2 week with agitation speed by 2 weeks in statistic state (p<0.001 ) and Cadmium content in untreated sate 6.543 mg/Lit after 2 weeks decreased to 2.005 and 1.902 mg/Lit respectively which prove that biomass of adsorbent can reduce significant toxic heavy metal but the potential of sage wastes are lower than black tea residues.
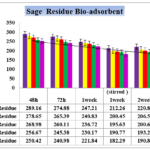
Figure 5. Effect of contact time on the removal of Nickel (initial Ni concentration=323.12 bio-adsorbent dose=0.1% ,0.2%,0.3%,0.4% and 0.5% W/V Viola Herbal tea residues in pharmaceutical Effluent , temperature=25 ± 2 ºC, agitation speed= 200 rpm), pH = 4.1.
The results of the increasing adsorbent to 0.5% in figure 5, showed significant differences in Nickel up -taking by bio-adsorbent after 48.But despite of other previous concentration it can be observed that passing time up to 1 week has no significant difference by 72 hours (p ≥ 0.05) and also 7 days (1 week) with stirring by 2 weeks without stirring too. The data showed that accomplishing of agitation speed= 200 rpm during 1 week and 14 days (2 weeks) with stirred have significant differentiate (p <0.05). Nickel content in untreated sate 323.1mg/Lit after 2 weeks decreased to 180.54 mg/Lit which has no difference by 0.5% concentration of biomass. This content of adsorbent can reduce 44.13% in pharmaceutical effluent which means removal by sage tea residue has less potential in comparison by black tea residue.
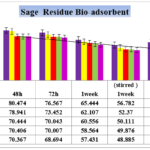
Figure 6- Effect of contact time on the removal of Cobalt (initial Co concentration=87.156 bio-adsorbent dose=0.1%,0.2%,0.3%,0.4% and 0.5% W/V Viola Herbal tea residues in pharmaceutical Effluent , temperature=25 ± 2 ºC, agitation speed= 200 rpm), pH = 4.1.
As Cobalt contents compared between the final results of 2 weeks contact time by 0.5 % bio-adsorbent (48.403 mg/Lit) to the untreated initial concentration (87.156 mg/Lit), the manifest removal trend was observed but not as much as bio-adsorbent of black tea residue.
Results in figure 6 in presence of 0.1%, 0.2%, 0.3%, 0.4% and 0.5% showed significant difference in Cobalt up -taking by bio-adsorbent after 48 by 72 hours contact time in any of studied contents of bio-mass of bio-adsorbents but at 72 hours and also 7 days (1 week) without stirring no significant differences between removal of heavy metal (p≥0.05).The data showed that accomplishing of agitation speed= 200 rpm during 14 days (2 weeks) without stirred have significant differentiate (p<0.02) just like lower concentrations of adsorbents in this study, but the potential of taking up Cobalt was increased significantly after 2 week with agitation speed reached the maximum rate of removal (p<0.001 ) .
Discussion
The results of current study revealed that cadmium, Nickel and Cobalt adsorption ranged from 43-88 % after agitation for different bio-adsorbents and also contact time and different doses regarding agitated states. On the whole all results by black tea residues has better results in detoxification of all studied heavy metals: Cadmium, Nickel and Cobalt although in this green ecofriendly and low cost method we did not use any chemical compounds as ligands and boosting higher efficacy. Results showed that low-cost and available bio- adsorbents can be fruitfully used for the removal of heavy metals with a very few concentration range of 0.1 up to 0.5 mg/lit. It also was found that the percentage removal of heavy metals was dependent on the chemical composition of tea residue as black tea has more potential rather than sage tea residues; dose and particle size of low cost adsorbent and adsorbent concentration. The contact time necessary for maximum adsorption was found to be 2 weeks. The optimum pH range for Cadmium, Cobalt and Nickel all studied in 4.1.
Results from this investigation revealed that Bio-adsorbent dose is one of the most important factors which affect significantly on influence specific uptake of all studied samples of wastewater effluent, but contact time was more effective factor significantly (p<0.05).Generally, for low bio-adsorbent dose, there is an enhanced heavy metals sorption for heavy metals Sorption capacity of different bio-adsorbents have been observed the significant effect based o chemical compounds and functional groups which working as ligands and bonding metals from pharmaceutical effluents.
Recently the utilization of many by-products or agricultural and Food industry wastes and also seaweeds, yeasts, and other dead microbial biomass for removal of heavy metals has been noticed [40-42]. Nowadays attention has been focused on the biomaterials which are byproducts or the wastes from large scale industrial operations and agricultural waste materials. The major advantages of bio-sorption over conventional treatment methods include: low cost, high efficiency, minimization of chemical or biological sludge, no additional nutrient requirement, and regeneration of bio-sorbents and possibility of metal recovery [21-24,28].Agricultural materials particularly those containing cellulose shows potential metal bio-sorption capacity [32, 43-47] . The basic components of the agricultural waste materials biomass include hemicellulose, lignin, extractives, lipids, proteins, simple sugars, water hydrocarbons, starch containing variety of functional groups that facilitates metal complexation which helps for the sequestering of heavy metals [48-50]. Agricultural waste mostly recommended as the materials being economic and ecofriendly due to their unique chemical composition, availability in abundance, renewable, low in cost and more efficient are seem to be viable option for heavy metal remediation.
Conclusion
The strong point of this environmentally green project was regeneration of bio-sorbent and recovery of metal ions and immobilization of the waste material for enhanced efficiency and recovery. Due to more consumption of herbal tea for medicinal properties it could be great achievement to reuse even the wastes and residue of them .One of the major advantage of current method is the applied adsorbent used in the experimental work is a home-made waste and also commercially available from coffee shops, most offices and even restaurants, and they are the waste of popular nonalcoholic beverage in many countries that could be collected in every house with no cost in fact. We highly recommend that to study the mechanism, which is necessary to have the exact information about the cell wall structure of the biomass as well as the solution chemistry and also other popular herbal teas such as Chamomile, Ginger, Rooibos, Lemon Balm and other ones.
Conflict of interest
None of the authors have any conflicts of interest associated with this study.
Acknowledgments
Nutrition & Food Sciences Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran-Iran (IAUMST) is gratefully acknowledged. This study has Non-grant fund.
References
- Ziarati P, Hochwimmer B, Lambert Brown D, Moradi M., Cruz –Rodriguez L. Breast Cancer disease and Heavy Metal: Cadmium as Key in “Medical Geology”. Gynecology and Women’s Health Care Journal 2020: 2(2); 1-13.
- Pourzare A, Ziarati P, Mousavi Z, Faraji AR . Removing Cadmium and Nickel Contents in Basil Cultivated in Pharmaceutical Effluent by chamomile (Matricaria chamomilla L.) Tea Residue. J Sci Discov, 2017; 1(1), jsd17006; DOI:10.24262/jsd.1.1.17006.
- Ahmadi M, Ziarati P, Manshadi M, Asgarpanah J, Mousavi Z. The Phytoremediation Technique for Cleaning Up Contaminated Soil by Geranium (Pelargonium roseum). Int J Farm & Alli Sci, 2013; 2 (15): 477-481.
- Ziarati P, Tosifi S. Comparison some physical and Chemical Properties of Green Olive (OLEA EUROPEA L.) In Iran Association with Ecological Conditions. International Journal of Plant, Animal and Environmental Sciences. 2014; 4(2): 519-528.
- Ziarati P, Azizi N. Consequences of Cooking Method in essential and Heavy Metal Contents in Brown and polished Alikazemi Rice. International Journal of Plant, Animal and Environmental Sciences. 2014; 4(2): 280-287.
- Yazdanparast S, Ziarati P, Asgarpanah J. Heavy Metals and Mineral Content and Nutritive Value of Some Iranian Manna. BBRA, 2014;11(2):1025-1029.
- Mirmohammad-Makki , Ziarati P. Determination of Histamine and Heavy Metal Concentrations in Tomato pastes and Fresh Tamato (Solanum lycopersicum) in Iran. BBRA, 2014;11(2): 537-544.
- Tavakoli-Hosseinabady B, Ziarati P, Ballali E, Umachandran K.Detoxification of Heavy Metals from Leafy Edible Vegetables by Agricultural Waste: Apricot Pit Shell. J Environ Anal Toxicol, 2018; 8 (1): 548. doi: 10.4172/2161-0525.1000548.
- Ziarati P, Mostafidi M, shirkhan F, Tamaskoni Zahedi M . ANALYSIS OF REMOVAL METHODS OF TOXIC HEAVY METALS USING BIO-ABSORBS. TECHNOGENIC AND ECOLOGICAL SAFETY, 2018; 4(2): 62-76. DOI: 10.5281/zenodo.1402587.
- Razafsha A, Ziarati P. Removal of Heavy Metals from Oryza sativa rice by sour lemon peel as bio-sorbent. Biomedical Pharmacol J. 2016; 9 (2): 739-749.
- Ziarati, P, MirMohammad-Makki F, Moslehishad M. Novel Adsorption Method for Contaminated Water by Wild Endemic Almond: Amygdalus scoparia. Biosciences Biotechnology Research Asia, 2016; 13(1): 147-153.
- Motaghi M, Ziarati P. Adsorptive Removal of Cadmium and Lead from Oryza sativa Rice by Banana Peel as Biosorbent. Biomed Pharmacology J, 2016; 9(2): 543-553.
- Ziarati P, El-Esawi M, sawicka B, Umachandran K, Mahmoud AED , Hochwimmer B, Vambol S, Vambol V. Investigation of Prospects for Phytoremediation Treatment of Soils Contaminated with Heavy Metals. J Med Discov , 2019; 4(2):1-16. jmd19011; DOI:10.24262/jmd.4.2.19011.
- Hochwimmer B , Ziarati P , Selinus O , Elwej A , Cruz-Rodriguez LD , Lambert Brown D , Zayas Tamayo AM , Moradi M , Cruz-Rodriguez L. A Predictive Geological Tool of Type 3 Diabetes (Alzheimer’s disease): The Polygonal Vortex Mineralisation Model a Medical Geology Perspective. Journal of Diabetes and Endocrinology Research.2020; 2(2): 1-15.
- Ziarati P, Mirmohammad Makki FS, Vambol S, Vambol V. Determination of Toxic Metals Content in Iranian and Italian Flavoured Olive Oil. Acta Technologica Agriculturae Journal. 2019; 22(2): 64–69. DOI: 10.2478/ata-2019-0012.
- Ziarati P, Mohsenin Moshiri I, Sadeghi P. Bio-adsorption of Heavy Metals from Aqueous Solutions by Natural and Modified non-living Roots of Wild Scorzonera incisa DC. J Sci Discov, 2017; 1(1), jsd17010; DOI:10.24262/jsd.1.1.17010.
- Ziarati P, Vambol V, Vambol S. Use of inductively coupled plasma optical emission spectrometry detection in determination of arsenic bioaccumulation in Trifolium pratense L. from contaminated soil. Ecological Questions 2020; 3(11): 1-11. Available from: http://dx.doi.org/10.12775/EQ.2020.003.
- Ziarati P, Davoudi, MHS, Kozub SM. et al. (2020). Sposib ochyshchennya vody [Method of water purification]. Ukrainian Patent NO. u 202002228 Kyiv: State Patent Office of Ukraine 2020.
- Ziarati P. Determination of some heavy metals in popular medicinal plants in Tehran’s market. Journal of Pharmaceutical and Health Sciences. 2012; 1(3): 31-36.
- Ziarati P, Mostafidi M, shirkhan F, Tamaskoni Zahedi M . ANALYSIS OF REMOVAL METHODS OF TOXIC HEAVY METALS USING BIO-ABSORBS. TECHNOGENIC AND ECOLOGICAL SAFETY, 2018; 4(2): 62-76. DOI: 10.5281/zenodo.1402587.
- Ziarati P. Determination of Contaminants in Some Iranian Popular Herbal Medicines . Journal of Environmental & Analytical Toxicology . 2012; 2 (1): 1.
- Ziarati, P, MirMohammad-Makki F, Moslehishad M. Novel Adsorption Method for Contaminated Water by Wild Endemic Almond: Amygdalus scoparia. Biosciences Biotechnology Research Asia, 2016; 13(1): 147-153.
- Motaghi M, Ziarati P. Adsorptive Removal of Cadmium and Lead from Oryza Sativa Rice by Banana Peel as Biosorbent. Biomed Pharmacology J. 2016; 9(2): 543-553.
- Ziarati P, Farasati Far B, Mashayekhi E, Sawicka B. Removing arsenic by food-processing waste (Zizyphus jujuba seeds) and study on its adsorptive properties. Technogenic and ecological safety. 2019; 5(1): 62–70. DOI: 10.5281/zenodo.2604648.
- Zahirnejad M, Ziarati P, Asgarpanah J. The Efficiency of Bio-adsorption of Heavy Metals from Pharmaceutical Effluent by Rumex crispus L. Seed. Journal of Pharmaceutical and Health Sciences. 2017; 5(3): 231-243.
- Ziarati P, Mostafidi M, Arabian S. et al. (2019). Patent № 138281 Ukraina, Sposib ochystky vody. Zayavka № u201904687; zayavl. 02.05.2019; opubl. 25.11.2019, Byul. № 22/2019.
- Ziarati P, Moradi D, Vambol V. Bioadsorption of heavy metals from the pharmaceutical effluents, contaminated soils and water by food and agricultural waste: a short review. Labour Protection Problems in Ukraine. 2020; 36(2): 3-7. DOI: 10.36804/nndipbop.36-2.2020.3-7.
- Ziarati P, Iranzad-Asl S, Asgarpanah J.Companion PELARGONIUM ROSEUM AND ROSMARINUS OFFICINALIS in Cleaning up Contaminated Soil by Phytoextraction Technique International Journal of Plant, Animal and Environmental Sciences. 2014; 4 ( 3) : 424-430.
- Ziarati P, Zolfaghari M, Azadi B. THE EFFECT OF TEA RESIDUE IN PROMOTING PHYTOREMEDIATION OF LAVANDULA ANGUSTIFOLI MILL.International Journal of Plant, Animal and Environmental Sciences.2014;(4) 2: 479-486.
- Ziarati P, Hochwimmer B. Reduction Nitrate Content from Contaminated Vegetable, by Hard Shell of Wild Endemic Almonds: Amygdalus lycioides and Amygdalus wendelboi. SciFed Drug Delivery Research Journal 2018; 2(2): 1-8.
- Mehrarad F, Ziarati P, Mousavi Z. Removing Heavy Metals from Pharmaceutical Effluent by Pelargonium Grandiflorum. Biomedical & Pharmacology Journal.2016; 9 (2): 151-161.
- Sobhani L, Ziarati P. Study on potential bio-adsorption of Tangerine peel in removal of heavy metals: Pb, Cd and Ni of vegetable coriander. J Sci Discov. 2017; 1(2): jsd17020; DOI:10.24262/jsd.1.2.17020.
- Shahsavan-Davoudi AH, Ziarati P. Green Method for Cadmium Removal from Pharmaceutical Effluent Laboratories by Grapefruit Peel. J Med Discov. 2020; 5(3):jmd20035; DOI:10.24262/jmd.5.3.20035.
- Bisset NG, Wichtl M. 2nd ed. CRC Press; Boca Raton, FI: 2001. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis with Reference to German Commission E Monographs; pp. 440–443.;
- Miura K, Kikuzaki H, Nakatani N Apianane terpenoids from Salvia officinalis. Phytochemistry. 2001; 58:1171–1175.; Physicians’ Desk Reference (PDR) for Herbal Medicines. 3rd ed. Thompson; Montvale, NJ: 2004. pp. 698–701.
- Arabian S, Ziarati P. Bioremediation of Heavy metal contaminated wastewater by herbal tea residue. Proceeding in 15th Iranian congress on Toxicology, 19-22 Nov 2019, page 136, Tehran-Iran.
- Garcia CSC, Menti C, Lambert APF. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): antioxidant, and antitumor in mammalian cells. An Acad Bras Ciênc. 2016; 88: 281–292.
- Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017; 7(4):433-440. doi:10.1016/j.jtcme.2016.12.014;
- 2000. Method 962.09. Official Methods of Analysis of AOAC International, 17th ed. 14 ed., AOAC International, Gaithersburg: Maryland USA, ISBN: 0935584676 9780935584677.
- Alidoost-Saharkhiz-Lahiji F, Ziarati P, JafarpourA . Potential of Rice Husk Biosorption in Reduction of Heavy Metals from Oryza sativa Rice, BIOSCIENCES BIOTECHNOLOGY RESEARCH ASIA. 2016; 13(4): 2231-2237.
- Gholizadeh E, Ziarati P. Remediation of Contaminated Rice Farmlands Soil and Oryza sativa Rice Product by Apple Pomace as AdsorbentRemediation of Contaminated Rice Farmlands Soil and Oryza sativa Rice Product by Apple Pomace as Adsorbent. BBRA . 2016; 13(4): 2245-2253.
- Ziarati P, Moslehisahd M . Determination of Heavy Metals (Cd, Pb, Ni) in Iranian and Imported Rice Consumed in Tehran. Iranian Journal of Nutrition Sciences & Food Technology. 2017; 12: 97-104.
- Jallilian Z, Ziarati P. High potential of Ferulago Angulate ( Schecht) Boiss in Adsorption of Heavy Metals. Biomed Pharmacol J. 2016; 9(1): 201-208.
- Shokri F, Ziarati P, Mousavi Z. Removal of Selected Heavy Metals from Pharmaceutical Effluent by Aloe Vera L. Biomedical Pharmacol J. 2016; 9 (2):705-713.
- Jafari A, Amin GR, Ziarati P. Potential of Echium ameonum Fisch & Mey in Removing Heavy Metals from Pharmaceutical Effluent Bioscience & Biotechnology Research Asia . 2016; 13(3): 1585-1594.
- Alimardan M, Ziarati P, Jafari Moghadam R. Adsorption of Heavy Metal Ions from Contaminated Soil by B. Integerrima Barberry. Biomedical Pharmacol J. 2016; 9 (1): 169-175.
- Yazdanparast S , Ziarati P, Asgarpanah J. Heavy Metals and Mineral Content and Nutritive Value of Some Iranian Manna. BBRA, 2014; 11(2):1025-1029.
- Hashem, A, Abdel-Halim, ES, El-Tahlawy, KF, Hebeish A. Enhancement of adsorption of Co (II) and Ni (II) ions onto peanut hulls though esterification using citric acid. Adsorp Sci. Technol .2005a.; 23:367–380.
- Hashem, A, Akasha RA, Ghith A, Hussein DA. Adsorbent based on agricultural wastes for heavy metal and dye removal: A review. Energy Edu. Sci. Technol. 2005b;19: 69–86.
- Sud D, Mahajan G , Kaur MP. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions – A review. Bioresource Technology. 2008; 99: 6017–6027. Available online at sciencedirect.com. doi:10.1016/j.biortech.2007.11.064.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/