J Med Discov (2019); 4(4):jmd19032; DOI:10.24262/jmd.4.4.19032; Received November 05th, 2019, Revised December 11st, 2019, Accepted December 20th, 2019, Published December 27th, 2019.
Fishing for answers: small zebrafish, great model for vasculogenesis and hematopoiesis
Xinyan Lu1,*
1Department of Human Genetics, University of Pittsburgh, PA, USA
* Correspondence: Xinyan Lu. Department of Human Genetics, University of Pittsburgh, PA, USA. Email address: xil215@pitt.edu.
Abstract
Zebrafish (Danio rerio) is a powerful model animal to study development, especially the processes of vasculogenesis and hematopoiesis. It has identity to vertebrates in genome sequence and conserved vessel formation and blood system development. The conventional forward large-scale genetic screening and recent reverse genome editing methods enable researchers to obtain combined methods in conducting the study of the vasculogenesis and hematopoiesis. In this review, the history of using zebrafish is reviewed by introducing the timeline of using zebrafish as a model organism and reviewing major contributions of researchers. Moreover, the power of this model as a tool to study vessel and blood development is discussed. In addition, the common methods and the resources of researching vasculogenesis and hematopoiesis are concluded. A summary of the standard operating system with a combined flow chart is presented for the comprehensive study for zebrafish users who plan to focus on vasculogenesis and hematopoiesis. Overall, the current study shows that zebrafish is acting as an ideal system to study the vascular and hematopoietic development and will continue to play a critical role in the investigation of vasculogenesis and hematopoiesis in the future.
Keywords: zebrafish; model organism, vasculogenesis, hematopoiesis
Introduction
With more than half centuries’ development, zebrafish has been in the recognition of a useful model organism in studying the formation of vessel and blood in embryonic development. This animal is a small tropical fish with a size of 2.5 to 4cm in length. The males and females are easy to distinguish by their coloring and body shape. Sexually matured zebrafish produce up to 300 embryos with proper controlling of temperature and feeding conditions. Standardized lab culturing systems enable enough oxygen for the first several days post fertilization (dpf).[1] Therefore, the easily-cultured system enables zebrafish embryos an ideal model for study embryonic development. In this review article, I briefly reviewed the major approach and resources used in the investigator, the development process of vessel and blood, the vascular and blood genetic mutant models used in the zebrafish.
Timeline of using zebrafish as a model organism
Zebrafish has been a key model animal in the research of development. Therefore, it is of great significance to understand the development of zebrafish study in a timeline matter. The first utilization of zebrafish as a model organism dated back to early 1960s. However, the benefit of using zebrafish as a model organism arises in attention by the pioneer scientist Dr. George Streisinger since 1972. Dr. Streisinger’s lab initiated the utilization of zebrafish by obtaining mutants by X-rays and Y-RAYS in pregonial germ cells. Compared with the mouse system, zebrafish is relatively easier in experimental manipulation. For example, zebrafish embryos display a strong surviving rate with proper temperature and feeding control.[2,3] The genetic knockdown and knockout approach are easier in manipulation due to enough embryos. Compared with the human system, the zebrafish genome has a seventy-percent similarity.[4] In addition, the manipulation in zebrafish avoids ethical issues in the study of embryogenesis and disease modelling. Therefore, the approach zebrafish study has shed light upon the investigation of the clinical research, serving the understanding of the investigation of the development process and the study of diseases. In the technical aspects, some assays are relatively inconvenient to conduct in biochemistry assays. For example, though some commercialized institutes and companies are contributing to the development of zebrafish antibody, there is still a lack of wide-use of efficient antibodies that recognize endogenous zebrafish proteins. Therefore, it is difficult to find the protein expression of certain genes of interest. In the early 1990s, Dr. Nüsslein-Volhard and Dr. Driever conducted genetic screening in zebrafish independently and revealed a series of mutants with phenotypes. However, forward genetic technical limitations existed and the genome sequence of zebrafish was not been fully sequenced by that time. In 2001, Wellcome Sanger Institute took the challenge and initiated the zebrafish genome project and revealed that the genome of zebrafish has over 1.5 billion base pairs with more than 26,000 protein-coding genes.
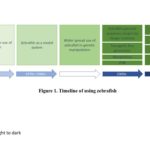
In the early 2000s, the method of studying zebrafish development also has a breakthrough by the introduction of more transgenic lines, which provide a convenient fluorescent marker of cells of interest. In 2000, the first zebrafish transgenic line generated was Tg(insulin:GFP) labeling beta cells, and similar transgenes were subsequently established that label endothelial cells Tg(fli1a:EGFP).[5,6] The wide use of transgenic and confocal laser scanning microscopy (CLSM) enable interest findings in vivo. Furthermore, the zebrafish community also learned skills from immunobiology and genomic biology. The use of Fluorescence-activated cell sorting (FACS) sorting has been applied in transgenic lines to sort single populations. Nowadays, a combined method of microarray, single-cell RNA sequencing (scRNA-seq) and genomic editing contribute to uncovering questions in a multi-dimensional way. In the genome editing process, investigators only used forward genetics to find out the genes which caused the phenotype in genetic mutants. When there were no ideal ways to have mutagenesis, researchers knockdowned (KD) genes of interest by morpholino (MO), which is an oligo developed by Genetools.[7] However, the off-target effect of morpholino has always been in controversy. Further genome editing methods of TALEN and CRISPR display the genetic mutants of zebrafish.[8,9] In 2003, some zebrafish experts started the Zebrafish Information Network (ZFIN) and provided a web-based resource including location for the curation and integration of zebrafish anatomical dictionary, gene expression, genomic sequence, gene ontology, collaborations and user input, which starts a new page in data sharing and community discussion.[10] Nowadays, the ZFIN website is still the most commonly used resource and the study of zebrafish is a combined process with multiple and interplay methods from different subjects. Figure 1 shows the timeline with significant events involved in the zebrafish study.
Fig 1. List of significant timelines of zebrafish study
After the initiation of using zebrafish in research in the 1960s, researchers developed methods to apply zebrafish as a model organism. During the 1980s to 1990s, the widespread use of genetic approaches makes the use of zebrafish broader. In the 2000s, the completion of genomic sequencing projects in zebrafish, multiple establishment of transgenic lines, genetic knockdown methods and the assemble of full information of zebrafish website ZFIN accelerate the impact of zebrafish in a new dimension. In the 2010s, increasing and advancing molecular and immune assays including knock out methods, RNA-sequencing, FACS have reformed the study of zebrafish to a new era.
After decades of development, zebrafish started to show its dominant advantages in the study of early embryo development, toxicity, neurogenesis, vasculogenesis, and hematopoiesis. [1,11-13] The zebrafish model is also unique because it overcomes some barriers in other species. For example, other model organisms are lethal due to lacking of blood flow. However, zebrafish showed that blood flow inhibition embryos could still be alive, enabling the model of “silent heart”.[14] With a closed circulation system, zebrafish displays its great advantages especially in the study of vascular and blood development.[1] The large-scale forward-genetic analysis and molecular biology and biochemistry assays have been applied in the zebrafish system successfully. Besides, zebrafish showed advantages in using the optical transparent embryos to obtain high-resolution imaging of blood vessels and blood cells. Recently, researchers used the zebrafish model and found a cure for a patient with advanced anomalous lymphatic disease unresponsive to conventional sirolimus therapy. The findings demonstrated that the mechanistic understanding and the knowledge of genetic classification helps to guide medical treatments with a model organism approach.[15] In addition to the developmental and vessel disease model, zebrafish also serves as a model organism for and malignant hematopoiesis. [16]
Vessel Development and Hematopoiesis in zebrafish embryogenesis
The anatomical structure of vasculature and blood cells are similar to vertebrates. The molecular regulatory mechanisms in vasculogenesis and hematopoiesis have high-level of conservation.[17-18] Hemangioblast is the precursor of both vascular endothelial cells and blood cells and was initially proposed in the chicken system in the last century and exists in the ventral mesoderm of zebrafish gastrula.[19] Early endothelial and blood cells express some common genes, including stem cell leukemia (scl) and vascular endothelial growth factor receptor 2 (flk1) in zebrafish.[20] Zebrafish genetic mutants, such as cloche, display strong defects in vessel and blood development, resulting in embryonic lethal.[21] Genes essential for blood and endothelial development contains the ETS binding sites. For example, the transcriptional factor, friend leukaemia integration 1 (fli1) acts at the top genetic hierarchy in driving the development of hematopoietic and endothelial cells.[22] ETS factors induce the expression of EC specific genes, such as flk1 and vascular endothelial cadherin (vecdn). The transcriptional factor scl and LIM domain only 2 (lmo2) act together in the process of hemangioblast in zebrafish.[23]
Vessel Development during zebrafish embryogenesis
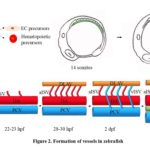
As an early step during embryogenesis, vessel development is critical for life. Vascular endothelial cells (ECs) are specified in the ventral mesoderm, where hematopoietic progenitors are also generated during early zebrafish embryogenesis (shown in Figure 2A). Over recent decades, there has been an increasing understanding of how the vasculature of zebrafish forms. As shown in Figure 2, there are two distinct mechanisms and steps in vessel forming, vasculogenesis (Figure 2A), and angiogenesis (Figure 2B). Vasculogenesis is a process of forming blood vessels. Through a de novo production of endothelial cells in the embryos, it gives rise to heart and primitive vascular plexus.[24] After the cells in the lateral plate mesoderm (LPM) become angioblast and then start migrating to the midline, vasculogenesis starts at 12 hours post-fertilization (hpf) [25] and the migration of angioblast to the midline, forming two major arteries and veins in the trunk region of zebrafish embryos, dorsal aorta (DA) and posterior cardinal vein (PCV) (shown in Figure 2A). [26,27] Arteries and veins form independent types of endothelial cells.[28,29] Ephrin B2 label arterial ECs and EPH Receptor B4 (EphB4) label venous endothelial cells. After vasculogenesis, a process named angiogenesis initiates, which is the formation of new vessels via sprouting and protrusion. The intersegmental vessels (ISV) and lumen genesis are formed in angiogenesis. Angiogenesis contributes to remodeling and expanding vessels in the vessel network.[24] As the first vessels formed in the angiogenesis process, ISVs sprout from the DA and run between each pair of the somite, and then connect to the dorsal longitudinal anastomotic vessel (DLAV) to transmit blood and plasma flow.[30] The identity of ISVs is controlled by the blood flow and potential signal pathways. Initially, all ISVs are arterial with no blood flow going through, and the remodeling transform into a balanced functional network with an equal number of arterial and venous ISVs. Recently, the ISV identity is demonstrated to be determined by the PCV origin on the finding that once an ISV is connected to the veins, the venous blood flow promotes the migration of endothelial cells, thereby causing the displacement of arterial endothelial cells by venous endothelial cells, completing the transformation of this arterial ISV (aISV) into the venous ISV (vISV).[14] Another independent group demonstrated that the “keep count” local pattern of the arterial and venous identity of ISVs is controlled by Notch signaling and mediated by the “fine-tunes” mechanism by adaptive blood flow. The in the trunk is pre-patterned by the activity of Notch signaling in the heterogeneous environment and controlled by the blood flow-induced regulation.[31] Finally, the newly formed ISVs sprout to the myoseptum in the trunk region and contribute to the structures of lymphatic vessels (LV). The formation of a balanced arterial and venous vessel and lymphatic vessels ensure the robust delivery of circulating blood cells, maintaining normal development of zebrafish in embryogenesis.
Fig 2. Vessel formation during zebrafish embryogenesis.
Vasculogenesis and angiogenesis process in embryogenesis. (A) The schematic picture shows that the ventral mesoderm region is the place where endothelial and hematopoietic precursor cells are generated. With the development of embryogenesis, vascuolgenesis processes from 14 somites to 16 somites. Green lines indicate the region where vasculogenesis occurs. (B) The model shows that the angiogenesis process of ISV sprouting in the trunk region of zebrafish embryos in a time-course matter.
Hematopoiesis during zebrafish embryogenesis
Hematopoiesis in vertebrates including zebrafish is a conserved genetic process which involves the differentiation of hematopoietic stem cells (HSCs) into all blood cells.[32] HSCs are multipotent and give rise to erythrocytes, neutrophils and other types of blood cells. Similar to the well-accepted major “two steps” hematopoiesis in the mouse and human, zebrafish hematopoiesis also comprises of primitive and definitive ones (Figure 3). Initially, primitive wave mainly contributes to erythrocytes production in the intermediate cell mass (ICM) and macrophages in rostral blood island (RBI) to provide oxygen and nutrition. However, the primitive hemopoiesis is temporary and transient and is quickly replaced by definitive hematopoiesis, thereby produceing HSCs.[33] HSCs in zebrafish depend on the expression of master regulators in the ventral wall of the aorta-gonad-mesonephros (AGM). Two major master regulators include runt-related transcription factor 1 (runx1) and c-myeloblastosis oncogene (cmyb). Runx1 plays key roles early in the specification of HSCs with distinguishing requirement roles of core bind factor β (CBFβ).[34] Cmyb acts with co-factor p300 to control the proliferation and differentiation of HSCs.[35] There is also a process that posterior blood island (PBI) starts from 24 hpf. PBI is thought to be seeded by AGM HSCs and plays an equivalent role in initiating definitive blood cell production. AGM is not the final residence for HSCs. Proliferating HSCs migrate to the caudal hematopoiesis tissue (CHT), generating more mature blood cells. Finally, HSCs travel to thymus, where they settle down during embryogenesis. The hematopoiesis organ in larval and adult zebrafish is the kidney.[18] In zebrafish, Notch signaling is required for the arterial identity, and the activated form of Notch (NICD) rescues the defects of HSC in the mindbomb mutants, which displays HSC deficiency.[36] Accumulating evidence showed that the fate of HSCs is determined by the Notch-Runx pathway.[37] Besides, some small non-coding RNAs are shown to be required for the process of hematopoiesis. For example, a miR-144–Krüppel-like factor d (klfd) pathway regulates the primitive hematopoiesis via the globin gene.[38] miR-126 and miR-142a-3p are essential for the definitive hematopoiesis during zebrafish embryogenesis.[39-41] Recently, the most abundant modification on eukaryote messenger RNA (mRNA) N6-methyladenosine (m6A) has also been shown to be required for the specification of HSCs in the embryogenesis of zebrafish and mouse. [42]
Fig 3. Timeline of hematopoiesis process during zebrafish embryogenesis.
Before 24 hpf, primitive hematopoiesis appears by the ICM-derived and RBI-derived matter. After the start of blood flow at 24 hpf, PBI acts as transient definitive hematopoiesis. Definitive HSCs emerge in the AGM region and then circulate through the vessels in the trunk and expand in the CHT region. After the transient stay in CHT region, the hematopoietic lineages appear in the kidney and thymus which are two major hematopoietic organs throughout the life span of adult zebrafish.
Endothelial to hematopoietic transition process in zebrafish
Hematopoietic cells and endothelial cells are closely related in the following aspects. They both share the same origin of hemangioblast; blood cells travel through the vessel tubes and generate shear stress which have effects on the vascular remodeling and reorganization; HSCs are maintained by the signals secreted by vasculature or vascular niche.[43] HSCs are specified and derived from a partial endothelial cells and this process is known as endothelial to hematopoietic transition process (EHT) (Figure 4). In this cell identity conversion, the hematopoietic transcriptional program is activated in a proportion in ventral wall aorta endothelial cells (VAE) and followed by morphology changes to round up cells adopting a blood cell fate.[44] Accumulating evidence showed multiple factors influence the EHT process. For example, thrombin receptor (F2r), which is a protease-activated G protein-coupled receptor, is demonstrated to be required for vascular development. F2r functions as a negative regulator of EHT process by imaging data.[45] On the other side, increased levels of adenosine increased the number of HSC in the dorsal aorta, and blocking the adenosine pathway showed decreased HSCs, demonstrating that Adenosine signaling is essential for the EHT process.[46]
Fig 4. Endothelial to hematopoietic transition process in zebrafish
Hematopoietic stem cells emerge from the existing endothelial cells lining the ventral wall of DA by a shape change from the flat-shaped endothelial cells to the rounded native HSCs from 26 to 48 hpf.
The approaches used in vessel and blood development in zebrafish
Zebrafish serves as an ideal model for the study of genetics and development. With recent decades of development in approaches, the methods used in the zebrafish system include most immunochemistry and immunostaining assays. Nowadays, there has been some standard protocols in the zebrafish system to answer the questions in the development of vessels and blood cells.
Table 1. Genetic mutants with vascular or/and blood defects
Mutant Name/allele |
Mutated genes |
Phenotype |
Mutant with both vessel and hematopoiesis defects |
||
Spadetail (spt) [52,53] |
tbx16 |
disorganized trunk somite and defect in ICM and AGM blood precursor, affect formation of ECs and hematopoietic cells |
Cloche (clo) [21,57] |
bHLH-PAS |
vascular and hematopoietic defects |
Vessel Mutant |
||
flk1t20257flk1t21588 [54] |
flk1 |
not required for vasculogeneisis and hematopoiesis, but affects sprouting angiogenesis |
etsrpy11 [55] |
etsrp |
ENU-induced mutant with defects in trunk angiogenesis |
after‐eight (aei),beamter (bea),deadly‐seven(des) [56,58] |
deltaDdeltaCnotch1a receptor |
defects in ISVpositioning and patterning |
Hematopoietic mutant |
||
Vlad tepes (vlt) [59] |
gata1 |
absence of erythrocytes |
Bloodless (bls) [60] |
unknown |
abnormal primitive hematopoiesis, normal definitive blood cells |
Moonshine (mon) [61] |
tif1g |
erythrocyte defect |
In situ hybridization
Whole-mount in situ hybridization (WISH) is used to detect the endogenous mRNA level in embryos. The advantage of is WISH is to allow the sites of expression of genes of interest. With decades of development, the digoxigenin-labeled antisense RNA probes serve as the major tool to detect the endogenous gene expression in embryos.[47] WISH is now widely used in finding out the gene expression and is usually the first step in a project, Therefore, there has been a common choice of vessel and blood cell markers based on the literature and information shared in ZFIN. Fli1a and flk1 are usually chosen to be endothelial markers; runx1 and cmyb are HSC markers. [48]
Gene Knockdown and Genetic mutants
Zebrafish was initially used in forward genetics and was applied in various genetic screens. The mutagenesis methods strengthened the study of zebrafish in genetic disease models. Investigators obtained genetic mutants via N-ethyl-N-nitrosourea (ENU). With the forward genetic search, people were able to find out the genes responsible for the mutation by positional cloning, which showed multiple genes responsible for the vessel and hematopoietic development, uncovering the genes with mutation in ENU sorted alleles.[48,49] In the early 2000s, genetic tools are absent for reverse genetics, i.e., mutagenesis of targeted genes. Therefore, the chemically synthesized oligomers morpholino (MO) antisense oligomers were widely used in microinjection to conduct gene knockdown (KD) in Xenopus and zebrafish. In the 2010s, the programmable site-specific mutagenesis methods including TALEN and CRISPR/Cas9 were developed in the zebrafish system. However, increasing findings from independent labs showed that genetics mutants in a small proportion of genes caused defects in embryogenesis. The phenomenon of genetic mutants hardly mimics the phenotypes of morpholino injected embryos.[50] Besides, morpholinos are easily caused side effects, especially apoptosis, making the study of apoptosis hard to investigate. Currently, researchers are still using morpholino, but more in a more rigorous manner combined with genetic mutants.[51]
Genetic mutants could be applied to find out whether genes of interest are expressed in vessels or blood. There are some other mutants available with both vessel and blood defects. Mutants with ventral mesoderm formation defect, spedetail, thereby affect the endothelial and hematopoietic cells formation.[52,53] Cloche mutants have both vascular and hematopoietic defects, failing to survive to adulthood.[21,53] If a gene of interest shows decrease or absence in either of clo or spt, it shows an indication that this gene is expressed in ECs or blood tissues. Because the expression of vascular and blood is hard to distinguish due to the close tissues, the use of genetics mutants serves as an ideal way to distinguish the expression in vessel and blood. Table 1 provides the commonly used genetic mutants with either vessel or blood defects. Whole-mount in situ could be applied to detect the expression of genes of interest. If the expression of a gene is absent or shows a dramatic reduction in vessel mutants, this gene is highly likely to be specifically expressed in vessels. The VEGF receptor 2 (VEGFR2) mutant reveal a specific disruption of angiogenesis.[54] The isolated ENU-induced mutants with the defects in the trunk angiogenesis turn out to be etsrp mutant.[55] In the three types of Notch mutants, they have angiogenic vascular defects in the trunk. Somite defects cause ISV mispositioning in Notch mutants.[56] By the same means, if a gene shows reduction or absence in hematopoietic mutants, it is highly expressed in hematopoietic tissues (Table 1). Therefore, a combination of multiple genetic mutants helps to understand the detailed expression pattern of genes of interest.
Transgenic reporter and Confocal Imaging
Due to the optical clarity in embryonic stages, zebrafish has become the most powerful vertebrate model system which is well suitable for the quantitative investigations with live-imaging. With the treatment of 1-Phenyl-2-thiourea (PTU), a drug inhibiting the growing of pigment cells, zebrafish embryos maintain transparent, thereby enabling imaging. It has been the goal of researchers to seek for high-quality images with high imaging speed, signal to noise ratio.[62] With the recent advanced in development of light microscopy technology including, confocal laser scanning microscope, multiphoton microscope, light sheet microscope, spinning disk microscopy, a comprehensive and protocols have been updated in studying the cellular dynamics during multiple processes, making the observation of vessel development, hematopoiesis and EHT process possible via confocal laser scanning microscope.
Table 2. Commonly used zebrafish transgenic lines
Transgenic Allele |
Labelled cells |
Tg(fli1a:EGFP)y1[5] |
all endothelial cells |
Tg(fli1a:EGFP)y7[48] |
labels all endothelial cell nuclei |
Tg(fli1a:pecam1-EGFP)ncv27 [64] |
endothelial cell junctions |
Tg(kdrl:ras-Cherry) s916 [65] |
endothelial cell membrane |
Tg(kdrl:EGFP)s843[26] |
endothelial cells |
Tg(flk1:nlsmCherry)is4[66] |
labels all endothelial cell nuclei |
Tg(flt1:tdTomato)hu5333 [67] |
arterial endothelial cells |
Tg(flt4:Citrine)hu7135[68] |
venous endothelial cells |
Tg(runx1P2:EGFP)zf188Tg(Mmu.Runx1:GFP)ioz1[63,69] |
definitive blood progenitors, HSCs |
Tg(cmyb:EGFP)zf169[70] |
definitive blood progenitors, HSCs |
Tg(gata1:dsRed)sd2[71] |
erythrocytes |
Tg(mpo:GFP)i113[72] |
neutrophils |
Tg(lysC:DsRed2)nz50Tg(lysC:EGFP)nz117[73] |
neutrophils |
Tg(rag2:dsRed)zf411[74] |
T lymphocytes |
Though in situ hybridization shows the pattern of expression at the level of mRNAs, transgenic lines help answer the questions of how endothelial and blood cells behave. For all endothelial cells, Tg(fli1a:EGFP)y1 is a widely-used transgenic in various manuscript in the zebrafish. To study the nuclei expression of ECs, Tg(fli1a:EGFP)y7 is a useful tool. Other transgenics including cell junctions, cell membranes contribute to the investigation of purposes of individuals. Combination of promoters of master regulator and fluorescence proteins enable multiple choices and is also a standard way to investigate the process of development. For example, a combination of endothelial transgenic line Tg(flk1:nlsmCherry)is4 and hematopoietic progenitor line Tg(Mmu.Runx1:GFP)ioz1 displayed hemogenic endothelial cells, providing the feasibility to observe the EHT process.[63] In addition, investigators used combination artery transgenic line Tg(flt1:tdTomato)hu5333 and vein transgenic lines Tg(flt4:Citrine)hu7135 to study the origins of artery or vein in the development of ISVs.[14] Table 2 provides the commonly used zebrafish transgenic lines in vessel and blood development. Additional resources could be found in ZFIN with updated information of fish resources.
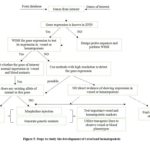
Zebrafish possess great advantages in being a model organism in the study of vessel and blood development. To make the operation to start a project with a gene of interest in vessel or blood development, Figure 5 shows a recommended workflow to perform the investigation of a gene. If an investigator initiates a project, genes of interest should be searched by either database including ZFIN or genes with specific expression in other species. If the expression of genes is provided in ZFIN or journals, the sequence of RNA probe design could be found. If the expression of genes is unknown, the investigators could apply online tools to design primers to make Ribo-probes and perform in situ hybridization and find out the expression. If the expression of genes of interest is very likely to be in either vessel or blood tissues, a further approach using the existing genetic mutants (Table 1) could be applied to find out the specific region where genes of interest express. For the gene function, morpholino injection and genetic mutants should be combined to test important markers of vessel and blood development. In addition, the combined use of available transgenic lines (Table 2) is recommended to study whether the loss of genes caused a defect in vessel development or hematopoiesis.
Fig 5. Flowchart to study the development of vessel and hematopoiesis in zebrafish embryogenesis
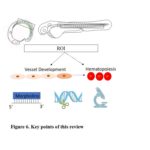
Fig 6. Key points of this review
Discussion
Though there has been some standard workflow for investigators to study the vasculogenesis and hematopoiesis by using zebrafish, challenges still exist. First, increasing evidence has shown that the phenotype of genetic mutants by short-piece deletion via CRISPR/Cas9 based genetic-editing approach show low levels of consistency with the phenotypes of morphants, including vessel and hematopoiesis abnormality. And it is found out some genetic mutants display an ectopic expression of inflammatory genes.[75] Deletion by CRISPR/Cas9 might cause offset effect by upregulation or compensation of other genes in genetic mutants and there is degradation from mutant RNA and these mutants fail to transcribe correctly and display mRNA decay. Therefore, it is highly suggested to design genetic mutant alleles by a way of minimizing transcriptional adaptation-derived compensation.[76] However, the methods of tuning gene compensation is still a challenge for most zebrafish users currently. Second, the conditional knock-out model in zebrafish is still in developing process. For the conditional knock out of general vessels, researchers could apply kdrl:Cre with LoxP system in conduct conditional knockout.[77,78] However, it still lacks of approaches to study specific vessel knock out in vessel development. In the hematopoietic system, conditional knock out approaches are still developing. Though there has been a recent research on the conditional mutagenesis by the loxP sites in zebrafish[79], most researchers rely on conventional knock out approaches in studying vasculogenesis and hematopoiesis. Third, it is also interesting that the process of EHT in zebrafish shares most similarities but also display some discrepancies with the mouse system. The definitive HSCs are generated from the ventral wall of dorsal aorta (DA) by a serious of signals and transcriptional factors in the zebrafish. However, the signals required for the zebrafish HSCs emergence have some discrepancies compared to the mouse HSC emergence, indicating that the two processes in zebrafish and mouse might be trigger by different potential regulators in the microenvironment or niche cells.[77,80]
Conclusion
In summary, the zebrafish acts as a well-accepted ideal system to study the vascular and hematopoietic development based on its multiple advantages. With the quick development and increasing support from the zebrafish community, the zebrafish system will maintain its pivotal roles in the investigation of vasculogenesis and hematopoiesis in the future study. As Figure 6 shown, researchers shall have a clear picture of the independent and interplaying roles in the understanding of the vessel and blood development in zebrafish embryogenesis. With further understanding of emerging and advanced approaches, investigators shall be able to explore and answer the specific scientific questions. Although challenges are presented in the exploration of scientific questions, the combined methods and existing resources will help accelerate the development of novel approaches, aiming to uncover more interesting and crucial questions in this field.
Conflict of interest
None
Acknowledgments
None
References
- Gore, A. V., Monzo, K., Cha, Y. R., Pan, W. & Weinstein, B. M. Vascular development in the zebrafish. Cold Spring Harb Perspect Med 2, a006684, doi:10.1101/cshperspect.a006684 (2012).
- Streisinger, G., Walker, C., Dower, N., Knauber, D. & Singer, F. Production of Clones of Homozygous Diploid Zebra Fish (Brachydanio-Rerio). Nature 291, 293-296, doi:DOI 10.1038/291293a0 (1981).
- Walker, C. & Streisinger, G. Induction of Mutations by Gamma-Rays in Pre-Gonial Germ-Cells of Zebrafish Embryos. Genetics 103, 125-136 (1983).
- Howe, K. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498-503, doi:10.1038/nature12111 (2013).
- Lawson, N. D. & Weinstein, B. M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248, 307-318, doi:10.1006/dbio.2002.0711 (2002).
- Huang, H., Vogel, S. S., Liu, N., Melton, D. A. & Lin, S. Analysis of pancreatic development in living transgenic zebrafish embryos. Mol Cell Endocrinol 177, 117-124, doi:10.1016/s0303-7207(01)00408-7 (2001).
- Nasevicius, A. & Ekker, S. C. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26, 216-220, doi:Doi 10.1038/79951 (2000).
- Zu, Y. et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods 10, 329-331, doi:10.1038/nmeth.2374 (2013).
- Irion, U., Krauss, J. & Nusslein-Volhard, C. Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development 141, 4827-4830, doi:10.1242/dev.115584 (2014).
- Sprague, J. et al. The Zebrafish Information Network (ZFIN): the zebrafish model organism database. Nucleic Acids Res 31, 241-243, doi:10.1093/nar/gkg027 (2003).
- Carradice, D. & Lieschke, G. J. Zebrafish in hematology: sushi or science? Blood 111, 3331-3342, doi:10.1182/blood-2007-10-052761 (2008).
- Hill, A. J., Teraoka, H., Heideman, W. & Peterson, R. E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86, 6-19, doi:10.1093/toxsci/kfi110 (2005).
- Stewart, A. M., Braubach, O., Spitsbergen, J., Gerlai, R. & Kalueffl, A. V. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 37, 264-278, doi:10.1016/j.tins.2014.02.011 (2014).
- Weijts, B. et al. Blood flow-induced Notch activation and endothelial migration enable vascular remodeling in zebrafish embryos. Nat Commun 9, 5314, doi:10.1038/s41467-018-07732-7 (2018).
- Li, D. et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat Med 25, 1116-+, doi:10.1038/s41591-019-0479-2 (2019).
- Jing, L. & Zon, L. I. Zebrafish as a model for normal and malignant hematopoiesis. Dis Model Mech 4, 433-438, doi:10.1242/dmm.006791 (2011).
- Isogai, S., Horiguchi, M. & Weinstein, B. M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev Biol 230, 278-301, doi:10.1006/dbio.2000.9995 (2001).
- Orkin, S. H. & Zon, L. I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 132, 631-644, doi:10.1016/j.cell.2008.01.025 (2008).
- Xiong, J. W. Molecular and developmental biology of the hemangioblast. Dev Dyn 237, 1218-1231, doi:10.1002/dvdy.21542 (2008).
- Kabrun, N. et al. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development 124, 2039-2048 (1997).
- Stainier, D. Y. R., Weinstein, B. M., Detrich, H. W., Zon, L. I. & Fishman, M. C. Cloche, an Early Acting Zebrafish Gene, Is Required by Both the Endothelial and Hematopoietic Lineages. Development 121, 3141-3150 (1995).
- Liu, F., Walmsley, M., Rodaway, A. & Patient, R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol 18, 1234-1240, doi:10.1016/j.cub.2008.07.048 (2008).
- Patterson, L. J. et al. The transcription factors Scl and Lmo2 act together during development of the hemangioblast in zebrafish. Blood 109, 2389-2398, doi:10.1182/blood-2006-02-003087 (2007).
- Patan, S. Vasculogenesis and angiogenesis. Cancer Treat Res 117, 3-32, doi:10.1007/978-1-4419-8871-3_1 (2004).
- Hogan, B. M. & Schulte-Merker, S. How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev Cell 42, 567-583, doi:10.1016/j.devcel.2017.08.015 (2017).
- Jin, S. W., Beis, D., Mitchell, T., Chen, J. N. & Stainier, D. Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199-5209, doi:10.1242/dev.02087 (2005).
- Lawson, N. D. & Weinstein, B. M. Arteries and veins: Making a difference with zebrafish. Nat Rev Genet 3, 674-682, doi:10.1038/nrg888 (2002).
- Torres-Vaazquez, J., Kamei, M. & Weinstein, B. M. Molecular distinction between arteries and veins. Cell Tissue Res 314, 43-59, doi:10.1007/s00441-003-0771-8 (2003).
- dela Paz, N. G. & D’Amore, P. A. Arterial versus venous endothelial cells. Cell Tissue Res 335, 5-16, doi:10.1007/s00441-008-0706-5 (2009).
- Childs, S., Chen, J. N., Garrity, D. M. & Fishman, M. C. Patterning of angiogenesis in the zebrafish embryo. Development 129, 973-982 (2002).
- Geudens, I. et al. Artery-vein specification in the zebrafish trunk is pre-patterned by heterogeneous Notch activity and balanced by flow-mediated fine-tuning. Development 146, doi:10.1242/dev.181024 (2019).
- Galloway, J. L. & Zon, L. I. Ontogeny of hematopoiesis: Examining the emergence of hematopoietic cells in the vertebrate embryo. Current Topics in Developmental Biology, Vol 53 53, 139-158, doi:Doi 10.1016/S0070-2153(03)53004-6 (2003).
- Davidson, A. J. & Zon, L. I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23, 7233-7246, doi:10.1038/sj.onc.1207943 (2004).
- Bresciani, E. et al. CBFbeta and RUNX1 are required at 2 different steps during the development of hematopoietic stem cells in zebrafish. Blood 124, 70-78, doi:10.1182/blood-2013-10-531988 (2014).
- Sandberg, M. L. et al. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell 8, 153-166, doi:10.1016/j.devcel.2004.12.015 (2005).
- Itoh, M. & Chitnis, A. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Faseb J 17, A995-A995 (2003).
- Burns, C. E., Traver, D., Mayhall, E., Shepard, J. L. & Zon, L. I. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev 19, 2331-2342, doi:10.1101/gad.1337005 (2005).
- Fu, Y. F. et al. Mir-144 selectively regulates embryonic alpha-hemoglobin synthesis during primitive erythropoiesis. Blood 113, 1340-1349, doi:10.1182/blood-2008-08-174854 (2009).
- Grabher, C. et al. Zebrafish microRNA-126 determines hematopoietic cell fate through c-Myb. Leukemia 25, 506-514, doi:10.1038/leu.2010.280 (2011).
- Lu, X. Y. et al. miR-142-3p regulates the formation and differentiation of hematopoietic stem cells in vertebrates. Cell Res 23, 1356-1368, doi:10.1038/cr.2013.145 (2013).
- Lu, X. Y., Wei, Y. L. & Liu, F. Direct regulation of p53 by miR-142a-3p mediates the survival of hematopoietic stem and progenitor cells in zebrafish. Cell Discov 1, doi:UNSP 1502710.1038/celldisc.2015.27 (2015).
- Zhang, C. et al. m(6)A modulates haematopoietic stem and progenitor cell specification. Nature 549, 273-276, doi:10.1038/nature23883 (2017).
- Khan, J. A. et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351, 176-180, doi:10.1126/science.aad0084 (2016).
- Bertrand, J. Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108-U120, doi:10.1038/nature08738 (2010).
- Yue, R. et al. Thrombin receptor regulates hematopoiesis and endothelial-to-hematopoietic transition. Dev Cell 22, 1092-1100, doi:10.1016/j.devcel.2012.01.025 (2012).
- Jing, L. et al. Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J Exp Med 212, 649-663, doi:10.1084/jem.20141528 (2015).
- Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3, 59-69, doi:10.1038/nprot.2007.514 (2008).
- Roman, B. L. et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 129, 3009-3019 (2002).
- Wei, Y., Xu, J., Zhang, W., Wen, Z. & Liu, F. RNA polymerase III component Rpc9 regulates hematopoietic stem and progenitor cell maintenance in zebrafish. Development 143, 2103-2110, doi:10.1242/dev.126797 (2016).
- Kok, F. O. et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell 32, 97-108, doi:10.1016/j.devcel.2014.11.018 (2015).
- Stainier, D. Y. R. et al. Guidelines for morpholino use in zebrafish. Plos Genet 13, e1007000, doi:10.1371/journal.pgen.1007000 (2017).
- Kimmel, C. B., Kane, D. A., Walker, C., Warga, R. M. & Rothman, M. B. A Mutation That Changes Cell-Movement and Cell Fate in the Zebrafish Embryo. Nature 337, 358-362, doi:DOI 10.1038/337358a0 (1989).
- Thompson, M. A. et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol 197, 248-269, doi:DOI 10.1006/dbio.1998.8887 (1998).
- Habeck, H. et al. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol 12, 1405-1412, doi:Pii S0960-9822(02)01044-8 Doi 10.1016/S0960-9822(02)01044-8 (2002).
- Pham, V. N. et al. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol 303, 772-783, doi:10.1016/j.ydbio.2006.10.030 (2007).
- Therapontos, C. & Vargesson, N. Zebrafish notch signalling pathway mutants exhibit trunk vessel patterning anomalies that are secondary to somite misregulation. Dev Dyn 239, 2761-2768, doi:10.1002/dvdy.22410 (2010).
- Reischauer, S. et al. Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature 535, 294-298, doi:10.1038/nature18614 (2016).
- van Eeden, F. J. et al. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development 123, 153-164 (1996).
- Lyons, S. E. et al. A nonsense mutation in zebrafish gata1 causes the bloodless phenotype in vlad tepes. P Natl Acad Sci USA 99, 5454-5459, doi:10.1073/pnas.082695299 (2002).
- Liao, E. C. et al. Non-cell autonomous requirement for the bloodless gene in primitive hematopoiesis of zebrafish. Development 129, 649-659 (2002).
- Ransom, D. G. et al. The Zebrafish moonshine gene encodes transcriptional intermediary factor 1 gamma, an essential regulator of hematopoiesis. Plos Biol 2, 1188-1196, doi:ARTN e23710.1371/journal.pbio.0020237 (2004).
- Keller, P. J. In vivo imaging of zebrafish embryogenesis. Methods 62, 268-278, doi:10.1016/j.ymeth.2013.03.015 (2013).
- Zhang, P. et al. G protein-coupled receptor 183 facilitates endothelial-to-hematopoietic transition via Notch1 inhibition. Cell Res 25, 1093-1107, doi:10.1038/cr.2015.109 (2015).
- Ando, K. et al. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 143, 1328-1339, doi:10.1242/dev.132654 (2016).
- Hogan, B. M. et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet 41, 396-398, doi:10.1038/ng.321 (2009).
- Wang, Y. et al. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development 137, 3119-3128, doi:10.1242/dev.048785 (2010).
- Bussmann, J. et al. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137, 2653-2657, doi:10.1242/dev.048207 (2010).
- van Impel, A. et al. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141, 1228-1238, doi:10.1242/dev.105031 (2014).
- Lam, E. Y. et al. Zebrafish runx1 promoter-EGFP transgenics mark discrete sites of definitive blood progenitors. Blood 113, 1241-1249, doi:10.1182/blood-2008-04-149898 (2009).
- North, T. E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007-1011, doi:10.1038/nature05883 (2007).
- Traver, D. et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol 4, 1238-1246, doi:10.1038/ni1007 (2003).
- Renshaw, S. A. et al. A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976-3978, doi:10.1182/blood-2006-05-024075 (2006).
- Hall, C., Flores, M. V., Storm, T., Crosier, K. & Crosier, P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. Bmc Dev Biol 7, 42, doi:10.1186/1471-213X-7-42 (2007).
- Willett, C. E., Cherry, J. J. & Steiner, L. A. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics 45, 394-404, doi:10.1007/s002510050221 (1997).
- Lai, J. K. H., Gagalova, K. K., Kuenne, C., El-Brolosy, M. A. & Stainier, D. Y. R. Induction of interferon-stimulated genes and cellular stress pathways by morpholinos in zebrafish. Dev Biol 454, 21-28, doi:10.1016/j.ydbio.2019.06.008 (2019).
- El-Brolosy, M. A. et al. Genetic compensation triggered by mutant mRNA degradation. Nature 568, 193-197, doi:10.1038/s41586-019-1064-z (2019).
- Bertrand, J. Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108-111, doi:10.1038/nature08738 (2010).
- Zhang, R. et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 498, 497-501, doi:10.1038/nature12322 (2013).
- Burg, L. et al. Conditional mutagenesis by oligonucleotide-mediated integration of loxP sites in zebrafish. PLoS Genet 14, e1007754, doi:10.1371/journal.pgen.1007754 (2018).
- Hamidi, S. & Sheng, G. Epithelial-mesenchymal transition in haematopoietic stem cell development and homeostasis. J Biochem 164, 265-275, doi:10.1093/jb/mvy063 (2018).
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/