J Med Discov (2019); 4(2):jmd19014; DOI:10.24262/jmd.4.2.19014; Received May 15th, 2019, Revised June 12nd, 2019, Accepted June 20th, 2019, Published July 18th, 2019
How to Deliver Cas9 into Brain
Honghao Bi1
1Department of Neurology,the Johns Hopkins University School of Medicine
* Correspondence: Honghao Bi, The Johns Hopkins University School of Medicine, Department of Neurology. E-mail: bihonghao@gmail.com.
Abstract
How to deliver therapy into brain remains a headache-causing question for hundreds of years. Even with modern gene therapy, the delivery through blood brain barrier is still challenging. Here, I reviewed recent development in gene therapies, especially CRISPR/Cas9, and their delivery method into brain. AAV9 and exosomes are introduced as the two most promising tools. By comparing the two systems, some advantages and disadvantages of each system were talked. I also raised several possible new directions in gene delivery research.
Keywords: Cas9, therapy, brain.
Introduction
Since Feng Zhang devised CRISPR/Cas9 system in 2012, people rapidly realized how powerful this genome-editing tool was[1]. Naturally, clinical gene therapy was gaining even more attentions. Several different general methods were proposed to insert genome-editing technology in gene therapy. Methods like directly editing embryo’s genes would face enormous ethical challenge and potential risks[2]. Instead, editing patients’ genes in non-germline cells by introducing CRISPR systems into target organs are more acceptable, both ethically and from risk considerations.
The introduction of CRISPR/Cas9 system into certain cells would require a safe and efficient delivery system. This challenge stimulated scientists’ inspiration and ambitions. In the past few years, different strategies were developed to deliver CRISPR/Cas9 or other genome-editing tools into cells. The strategies are generalized into two categories: viral and non-viral delivery system.
Viral delivery system takes advantage of viral infection ability and insert genome editing fragment into viral genome. The genome editing tools will be taken into cells as virus infecting cells and expressed in the cells, which in turn will edit target genes. Common viruses used in delivery include Adeno-associated virus (AAV) and Lentivirus. Viral delivery system was devised under the idea “one and done” treatment style. Instead of multiple injections in non-viral delivery system, viral delivery system tried to achieve the treatment goal by one shot. Supposedly the virus would replicate and infect human cells and finally achieve our goals.
On the other side, non-viral delivery system used chemical or physical measures like electroporation, microinjection, or nanoparticles to introduce DNA, RNA or proteins into target cells. Comparing to viral delivery system, non-viral delivery system has less safety concerns and larger capacity.
The delivery to central nerve system (CNS) raised additional challenges due to the existence of blood brain barrier (BBB). AAV becomes the most prominent virus used in brain gene editing, as it is capable of crossing BBB. The non-viral strategy usually requires special measures like brain injection or pre-natal processing[3-5]. Such operation causes obstacles in future clinical usage. In my personal opinion, breaking through BBB with physical force or deliver before BBB is fully developed does not count as going through BBB. This leaves AAV our only candidate to penetrate through BBB.
However, I do want to talk about exosome as a potential measure to deliver CRISPR/Cas9 into brain. Exosomes are 30-100nm vesicles secreted by all kinds of cells. The small vesicles can bear limited number of molecules including proteins and miRNAs. A hybrid of exosome and liposome was proven capable of delivering larger fragment like CRISPR/Cas9 system into mesenchyme stem cells (MSCs)[6]. Exosomes are also known to regulate brain functions when taken up by neurons. Taken together, it is reasonable to consider exosome as a potential candidate to deliver gene-editing tools to brain.
Adeno-associated virus (AAV) delivery and gene editing therapy
AAVs are single strand DNA dependoviruses that only infect human with the existence of viruses like adenoviruses or herpes simplex viruses (HSVs). Without the helper viruses, AAVs would go through a lysogenic cycle and integrate its genome fragment into Chromosome 19[7,8]. Under the lysogenic cycle, AAVs have limited gene replication and expression, which makes it with low infectivity and safe as delivery system.
Invasive administrations like Intraparenchymal (IPa), Intrathecal (IT) or Intracerebroventricular (ICV) were used in the earlier times and successfully delivered genes into CNS. Later, intravenous (IV) delivery of AAVs was proven capable to deliver genes into CNS, providing AAVs capability to cross BBB[9,10].
The idea of delivering genes into CNS by systemic injection is certainly attracting. Among the 12 serotype AAVs, AAV2 is most studied and has been used in multiple clinical trials, while AAV9 displayed the greatest ability to penetrate BBB[11,12]. By systemic injection, AAV9 is observed with the highest distribution in CNS, as well as tropism in liver, heart and skeletal muscle. Although AAVrh.10 was also reported later to have extensive distribution in brain, AAV9 remains the most studied AAV in CNS delivery. IV (intravascular) injection or rAAV9 (recombinant AAV9) is capable to rescue most of ASPA deficiency by delivering ASPA gene into CNS[13,14]. IV rAAV9 mediated therapy for spinal muscular atrophy has also been entered into clinical research and showed promising results[15].
The distribution of AAVs is determined by the penetration of AAVs and its distribution in the system. Besides increasing the transduction strength, research into decreasing peripheral accumulation and off-target toxicity was conducted in parallel. Strategies including tissue specific promoters, miRNA inhibition by inserting miRNA recognition site in 3’ UTR, and capsid engineering have been used to decrease peripheral targeting activity. Recently, Wang etc. reported a capsid engineered AAV9 that can significantly reduce transduction in peripheral tissues[16].
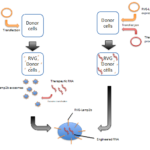
Fig.1 Methods to deliver RNA cargoes into exosomes. The RNAs can be delivered either by exosome transfection or through natural method by expressing therapeutic RNA in donor cells.
Fig 2. Splitted Cas9 delivered into CNS by exosome
The idea to treat CNS disease in one IV injection is irresistible, although certain disadvantage cannot be ignored in AAV mediated therapy. The maximum capacity of AAV is around 4.7kb, which limits its usage in gene editing therapy (spCas9 is 4.2kb). Also, AAVs showed a delay in gene transduction. Because the AAVs need a second-strand synthesis, the vector would need ~2 weeks to reach maximum expression. Although it could be corrected by using double stranded AAVs, the double strand method would in turn reduce capacity to 2.2kb[16]. Also, AAV9 distributes in multiple tissues besides CNS, and its accumulation in CNS is largely determined by total viral load and organ distributions. This would inevitably cause non-specific toxicity in systemic injections.
Exosome mediated gene delivery
Exosomes are small extracellular vesicles secreted by all kinds of cells in human body. Initially people thought exosomes were just garbage, but the following researches showed that exosomes function as transporters between cells carrying various cargoes both inside and on the surface of the vesicles[17]. Besides the lipids and proteins carried on its surface, exosomes also contain macromolecules like lipid, protein and RNA. The composition of exosomes cargo reflects their parental cells state. The secreted exosomes can selectively enter certain cells by endocytosis through certain cell surface receptors. In a recent report, exosomes endocytosis was shown mediated by cell filopodia. The filopodia “grab and drag” extracellular exosome to the endocytic hot spot on cell surface and internalize the exosome. After internalization, the exosomes mainly move and stop in ER region, suggesting its function in post-transcriptional regulation[18].
Exosomes are exempt from immunity, opsonins, coagulation factors, and capable to penetrate BBB. All the characteristics make exosome a perfect tool for drug delivery into CNS. By incubation, electroporation, or sonication, drugs can be delivered into exosomes and administered as exosome vehicle drug. For example, co-incubation of crucumin with exosome can produce crucumin-exosome, which was proven to alleviate cell junction proteins[19]
The RNA cargo of exosomes can be delivered either by exosome transfection or natural pathway (Fig. 1). Exosomes are natural miRNA carriers protecting miRNAs from RNase[20,21]. As miRNAs are gaining attentions in gene therapy, scientists began to engineer exosomes into miRNA delivery tools. Ohno etc. reported that by engineering exosomes to carry GE11 or EGF (epidermal growth factor), proteins specifically combining to EGFR (epidermal growth factor receptor), miRNA let-7a was successfully delivered into breast cancer cells and inhibit tumor growth in vivo[22].
Table 1. Current clinical trials with AAV9 (Data is from ClinicalTrials.gov)
| ClinicalTrials.gov Identifier | Study Title | Conditions | Interventions | Administration route | Starting date | Phase |
| NCT03770572 | Gene Transfer Study of AAV9-CLN3 for Treatment NCL Type 3 | CLN3-Related Neuronal Ceroid-Lipofuscinosis | AAV9-CLN3 | IT | 10-Dec-18 | Phase1, Phase2 |
| NCT03952637 | Intravenous Gene Transfer With an AAV9 Vector Expressing Human <=-Galactosidase in Type II GM1 Gangliosidosis | Lysosomal Diseases | AAV9-GLB1 | IV | 16-May-19 | Phase1, Phase2 |
| Gangliosidosis | ||||||
| GM1 | ||||||
| NCT03882437 | Gene Therapy for Male Patients With Danon Disease Using RP-A501; AAV9.LAMP2B | Danon Disease | RP-A501 | IV | 20-Mar-19 | Phase1 |
| NCT02240407 | Re-administration of Intramuscular AAV9 in Patients With Late-Onset Pompe Disease | Pompe Disease | Recombinant Adeno-Associated Virus Acid Alpha-Glucosidase |
IM (intramuscularly) |
15-Sep-14 | Phase 1 |
| NCT02725580 | Batten CLN6 Gene Therapy | Batten Disease | scAVV9.CB.CLN6 | IT | 1-Apr-16 | Phase1, Phase2 |
| CLN6 | ||||||
| NCT03381729 | Study of Intrathecal Administration of AVXS-101 for Spinal Muscular Atrophy | Spinal Muscular Atrophy | AVXS-101 | IT | 22-Dec-17 | Phase 1 |
| NCT02122952 | Gene Transfer Clinical Trial for Spinal Muscular Atrophy Type 1 | Spinal Muscular Atrophy 1 | AVXS-101 | IV | 25-Apr-14 | Phase 1 |
Besides miRNA, siRNA was also proven eligible to be delivered by exosomes. Alvarez-Erviti etc. used electroporation to load siRNAs into modified exosomes and successfully knocked down target genes in specific CNS tissues. The modified exosomes were equipped with CNS specific RVG-Lamp2b (rabies viral glycoprotein fused Lamp2b protein, Lamp2b is a membrane protein of exosomes), which shows highly selection to accumulate exosomes in CNS[22].
Due to strict limit of load volume, exosomes were usually considered not capable to carry CRISPR/Cas9. Lin etc. used exosome-liposome hybrid nanoparticles to contain CRISPR/Cas9 plasmid and successfully delivered CRISPR/Cas9 system in mesenchymal stem cells (MSCs)[6]. It is still not reported whether this hybrid nanoparticle can deliver CRISPR/Cas9 system into brain.
Future directions
rAAVs have shown promising potential in delivering genes into CNS, with AAV2 and AAV9 already entered into clinical trials (Table 1). Exosomes, on the other hand, are now a new star in CNS drug delivery.
Besides the advantage of less risk in immunity reactions, exosomes also show highly tissue selection by fusing different signals with Lamp2b on the membrane. The highly specificity provides treatment method with less potential side effect and non-specific toxicity.
However, the small loading size seriously limits exosomes use in gene editing therapy. Methods like exosome-liposome seem to be a good idea to overcome this disadvantage, although it is not sure whether the large vesicle can cross BBB.
Besides increasing load size, a split-Cas9 system reported in 2015 might also provide a solution. The system used split and fused Cas9 protein with FK506 binding protein 12 (FKBP) and FKBP rapamycin binding (FRB) domains. Such design was intended to control Cas9 activity, but it would also reduce Cas9 plasmid size[23,24]. It is possible to use the split version Cas9 in exosomes and perform gene editing in the brain (Fig. 2).
The combination of different technologies, like combined use of rAAV9 and exosomes, is also an interesting topic. Providing that rAAV9 can bear Cas9 and exosomes can target specific organs, combination of two different tools might lead a good strategy to conduct gene editing in brain. For example, using AAV9 to express Cas9 protein in the brain and later transducing guiding RNAs with exosomes specifically in brain. Or use exosomes to deliver miRNAs or siRNAs inhibiting AAV9 in peripheral organs.
Conclusion
AAVs and exosomes are the two most prevalent tools used in gene therapy delivery. AAVs are especially attractive for its one shot and all effect. The replication of virus would finally reach a maximum effect of treatment. However, AAVs also face challenge in safety and non-specific toxicity. Exosomes, on the other side, can overcome the disadvantages of AAVs, but are difficult to prepare and require multiple shots to achieve treatment goal. Both tools show great potential and we expect more tools developed in the future.
Conflict of interest
None
Acknowledgments
None
References
1. Cong, L. et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science (80-. ). 339, 819–823 (2013).
2. Bi, H. Technique discussion of CRISPR babies –A comment to Jiankui He’s research. J Med Discov 3, (2018).
3. Zuckermann, M. et al. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat. Commun. 6, 7391 (2015).
4. Straub, C., Granger, A. J., Saulnier, J. L. & Sabatini, B. L. CRISPR/Cas9-mediated gene knock-down in post-mitotic neurons. PLoS One 9, e105584 (2014).
5. Lee, B. et al. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat. Biomed. Eng. 2, 497–507 (2018).
6. Lin, Y. et al. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger. 5, 1700611 (2018).
7. Kay, M. A. et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 24, 257–261 (2000).
8. Kotin, R. M. et al. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. U. S. A. 87, 2211–5 (1990).
9. Foust, K. D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 (2009).
10. Duque, S. et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 17, 1187–96 (2009).
11. Yang, B. et al. Global CNS Transduction of Adult Mice by Intravenously Delivered rAAVrh.8 and rAAVrh.10 and Nonhuman Primates by rAAVrh.10. Mol. Ther. 22, 1299–1309 (2014).
12. Zhang, H. et al. Several rAAV Vectors Efficiently Cross the Blood–brain Barrier and Transduce Neurons and Astrocytes in the Neonatal Mouse Central Nervous System. Mol. Ther. 19, 1440–1448 (2011).
13. Ahmed, S. S. et al. A Single Intravenous rAAV Injection as Late as P20 Achieves Efficacious and Sustained CNS Gene Therapy in Canavan Mice. Mol. Ther. 21, 2136–2147 (2013).
14. Gessler, D. J. et al. Redirecting N-acetylaspartate metabolism in the central nervous system normalizes myelination and rescues Canavan disease. JCI insight 2, e90807 (2017).
15. Mendell, J. R. et al. 480. Gene Therapy for Spinal Muscular Atrophy Type 1 Shows Potential to Improve Survival and Motor Functional Outcomes. Mol. Ther. 24, S190 (2016).
16. Wang, D. et al. A Rationally Engineered Capsid Variant of AAV9 for Systemic CNS-Directed and Peripheral Tissue-Detargeted Gene Delivery in Neonates. Mol. Ther. Methods Clin. Dev. 9, 234–246 (2018).
17. Jiang, X.-C. & Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 521, 167–175 (2017).
18. Heusermann, W. et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 213, 173–184 (2016).
19. Kalani, A., Kamat, P., Chaturvedi, P., Tyagi, S. & Tyagi, N. Curcumin-Primed Exosomes Mitigate Endothelial Cell Dysfunction during Hyperhomocysteinemia. Life Sci. 107, 1 (2014).
20. Pegtel, D. M. et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. U. S. A. 107, 6328–33 (2010).
21. Bryniarski, K. et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J. Allergy Clin. Immunol. 132, 170–81 (2013).
22. Ohno, S. et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 21, 185–191 (2013).
23. Zetsche, B., Volz, S. E. & Zhang, F. A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat. Biotechnol. 33, 139–142 (2015).
24. Wright, A. V. et al. Rational design of a split-Cas9 enzyme complex. Proc. Natl. Acad. Sci. 112, 2984–2989 (2015).
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/