J Med Discov (2019); 4(2):jmd19011; DOI:10.24262/jmd.4.2.19011; Received May 9th, 2019, Revised May 27th, 2019, Accepted May 31st, 2019, Published June 2nd, 2019
Investigation of Prospects for Phytoremediation Treatment of Soils Contaminated with Heavy Metals
Parisa Ziarati1*, Mohamed El-Esawi2, Barbara Sawicka3, Krishnan Umachandran4, Alaa El Din Mahmoud5, Bernhard Hochwimmer6, Sergij Vambol7, Viola Vambol7
¹Nutrition & Food Sciences Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran-Iran
2Botany Department, Faculty of Science, Tanta University, Tanta, Egypt
3Department of Plant Production Technology and Commodities Sciences, Faculty of Agrobioengineering, University of Life Sciences in Lublin, Poland
4General Manager – Org. Dev., NELCAST Ltd., 159, TTK Road, Alwarpet, Chennai – 600018, India
5Environmental Sciences Department, Faculty of Science, Alexandria University, 21511 Alexandria, Egypt
6Associates Pty Ltd, Consultant Geologists: Mineral Exploration; Mining; Environmental Geology, Albury, Australia
7Berdyansk State Pedagogical University, Berdyansk, Ukraine
* Correspondence: : Parisa Ziarati, Nutrition & Food Sciences Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran-Iran .No 99, Yakhchal, Gholhak, Dr. Shariati, Tehran-Iran. Tel: +98-21-22600037; Fax: +98-21-22633986. E-mail: ziarati.p@iaups.ac.ir
Abstract
Phytoextraction or immobilization is more cost-effective in the field of removal of heavy metals from contaminated soils. These techniques have fewer side effects than physical and chemical techniques. It is the main and most promising technique, in situ or ex situ treatment/removal of contaminated soils, sediments and water. The use of plants as phytoremediators reduces the amount of inorganic components of soil impurities (heavy metals). The main aim of current study is focus on plants which could accumulate large amounts of these pollutants, but are also characterized by high growth rates, tolerate the toxic effects of heavy metals, and mostly adapted to the local environment and climate. Besides they must be resistant to pathogens and pests, easy to grow and exclude the possibility of contamination of animals and humans through the food chain. This study provides a systematic review of publications related to advances in the phytoremediation treatment of contaminated soils, and briefly explains the current limitations in the use of plants to removal heavy metals from the contaminated soil. The main limiting factor is the entry of heavy metals into animals and humans through the food chain. Another limiting factor is the possible negative impact on the genetics of plants used for soil treatment, because this may facilitate to the extinction of this species plants. The investigation showed that the questions of the influence of heavy metals on plant genes deserve serious attention. This is important to prevent reduce species diversity in nature. However, today these issues are very little studied.
Keywords: phytoremediation; immobilization; heavy metals; degradation.
Introduction
There are different types of Phytoremediation methods – Chemical, Biological and Physical. Phytoremediation includes the processes of contaminant removal (Table 1). The premise for inclusion of toxicity assessment to control the degree of environmental pollution has become evidence that the study of the presence and elimination of chemical compounds based solely on the measurement of their concentration may not mean a real improvement in the quality of the environment [1].
While using increased phytoremediation the ecological characteristics of the plants used should be considered. If contaminant is taken up by the plant, then this fact facilitates entry of the contaminant into the food chain. Diverse communities of plants increase total resource use and enhance nutrient cycling on a seasonal complementarity timescale. Phytoremediation may be used as a short-term treatment option to rehabilitate soils for residential or commercial development, or even for creation of a more sustainable wildlife habitat.
We see our mission in an attempt to study current advances in the field of phytoremediation treatment of contaminated soils, and understand and briefly explain the existing factors, which limit the use of plants to remove heavy metals from the soil. Consequently, in the course of our research it is necessary to clarify several questions:
Firstly, at what pollution is possible the use of phytoremediation treatment and what plants are used for this?
Secondly, what happens to a plant when it absorbs heavy metals: does it get into the food chain or it dries out and can no longer recover?
Thus, the main aim of current study is focus on plants which could accumulate large amounts of heavy metal pollutants, but are also characterized by high growth rates, tolerate the toxic effects of heavy metals, and mostly adapted to the local environment and climate. Besides they must be resistant to pathogens and pests, easy to grow and exclude the possibility of contamination of animals and humans through the food chain.
Table 1. Phytoremediation process and mechanisms in contaminant elimination
| No. | Process | Mechanism | Contaminant |
| 1 | Rhizofiltration | Rhizosphere accumulation | Organics/Inorganics |
| 2 | Phytostabilisation | Complexation | Inorganics |
| 3 | Phytoextraction | Hyper-accumulation | Inorganics |
| 4 | Phytovolatilization | Volatilization by leaves | Organics/Inorganics |
| 5 | Phytotransformation | Degradation in plant | Organics |
Phytoextraction
1.1 Physical and chemical techniques – in situ or ex situ treatment.
It is assumed that the sensitivity of the ecosystem depends on the most sensitive species, and the protection of the ecosystem structure will help to protect the functioning of biocenoses. Environmental risk assessment is defined as the process of assessing the probability that adverse environmental effects may occur as a result of exposure to one or more stressors. Stressors include physical, chemical and biological factors that cause adverse effects in organisms, in populations, teams, biocenoses and ecosystems [1]. The risk can be assessed if:
- the stressor has the natural ability to cause one or more negative effects, its occurrence in contact or co-occurrence with ecological components (organisms, populations, syndromes, biocenoses, ecosystems) is respectively long and in sufficient intensity to produce a harmful effect;
- the environmental risk assessment may be performed for one or more stressors and ecological components and is an aid in the identification of ecological problems, indicating priority actions and constituting scientific grounds for taking legal regulations [2-7].
1.2 The role of anthropogenic activity in the accumulation of heavy metals
Heavy metals are widely distributed in soil through fertilizer, natural weathering erosion, excretory disposals of animals and include urine, feces, and milk [8]. A particularly powerful source of heavy metals is the anthropogenic activity: the accumulation and decomposition of waste in a landfills [9-12, transport emissions [13-16], heavy metal emissions from industry [17-22], etc. Moreover, the depth of penetration into the soil depends on its properties and can be pre-predicted [23-24].
In the process of phytoremediation of heavy metals from the environment, individual plants accumulate them in their roots. This occurs until a satisfactory level of total pollution is achieved. The bio-mass by natural process induces the metal transfer to forming plant biomass and concentrates the pollutant by microbiologic composting or thermal burning of chemical while in the induced phytoextraction chelate application of the soil happens [25].
One of these heavy metals is cadmium which is extensively applied and particularly toxic in comparatively low dosages [26]. It has anonymous vital purpose in higher plants, is effortlessly absorbed by plant roots and transported to the shoots, hence the danger assessment of food chain infection is convoluted by the absence of a dependable connection among soil Cd and crop Cd [27].
1.3 Phytoremediation as an indispensable tool in ecological management
Heavy metals get into plants through roots, where the contaminated soils pollute groundwater and aquifers. Such plants when consumed by human or animals lead to ingestion [28-30]. Phytoremediation is an essential tool in ecological management. Evaluating remedial alternatives of polluted soils is an important function in achieving management goals effectively [31-32].Phytoremediation comprises of two uptake processes which has a reversible process called biosorption, and an irreversible, ion sequestration named as bioaccumulation [33].
Phytoremediation methods - Chemical, Biological and Physical
Application of genetic engineering technology makes efficient remediation plants and microbes develop microbe-plant combination systems. Cost effective remediation works on wide range of contaminated sites that are almost on the soil surface, comparatively non-leachable, and have greater area coverage.
Companion planting from pollutant source and segregated microbial, cellular, whole live plant and plant waste food and agricultural, forestry wastes can also have a role in relieving more serious metal pollutants in mining situations, and simultaneously relieve pollutant pleasures from remediated soils that can be returned to agriculture or ecological roles.
This in turn enhances “social license to operate” for industry and mining groups more effectively.
The practical application of phytoremediation promotes the soil resources for soil remediation and protects the biofuel crop production bringing revenue to the owners of contaminated sites [32]. Certain plants near metal mines thrived in heavily metal-contaminated soils, hence knowledge about different plant species which absorb and transport metals under different conditions are to be appropriately chosen for phytoremediation of the polluted regions [34].
2.1 Phytoremediation unsuitable in certain regions
Toxicity half-life depends on the toxic compound. The half-life, i.e. the half-life of the transformation, is the time during which half of the starting amount of the substrate will undergo the transformation (reaction). In the case of a radioactive transformation, proceeding according to the first-order reaction equation, the half-time of the transformation is constant, independent of the amount (content) of the radioactive isotope, which allows to calculate the residue after 0.5n transformation periods as 0.5n, e.g. after 4 t 0.5 each will remain 0.54 or 1/16 (about 6%) of the initial amount of substrate (radioactive isotope). Moniczewski et al. [35] report that the half-life of lead lasts over 20 years. Government agencies have set limits only for certain metals (e.g. for cadmium, arsenic, lead), while for others, i.e. cobalt, chromium, nickel, regulation of this type is still missing [35].
The results obtained in 2008 by Małachowska-Jutsz [31] have shown that the interdependence between plants and fungi is important in soil phytoremediation processes. This author has developed a method of intensifying phytoremediation of soils contaminated with petroleum compounds using mycorrhizal fungi (phytoremediation + bioaugmentation). In her research, she evaluated the processes of hydrocarbon degradation in soils, where phytoremediation was carried out using an inoculum based on autochthonic strains of microorganisms in relation to soils subjected to phytoremediation processes and self-cleaning. It was shown that in soil contaminated with hydrocarbons, colonization of plant roots by mycorrhizal fungi had a positive effect on the growth and development of roots as well as on the distribution of pollutants by other organisms of the rhizosphere. The efficiency of hydrocarbon elimination from the soil depended on the variant of the phytoremediation method used, i.e. the type of plant and biopreparation (bacterial or fungal). It has been shown that the best results can be obtained in the bioremediation process using a monocotyledonous plant and inoculants of mycorrhizal fungi isolated from contaminated soil. It turned out that the symbiosis of mycorrhizal fungi with monocotyledonous plants increases the efficiency of 2-, 3-, 4- and 5- ring hydrocarbon removal by ca. 40%, and 6-ring mycelium by ca. 30% in comparison with the samples in which plants without inoculation of mycorrhizal fungi [31].
Phytoremediation has an integral role to reduce risks in these future time scales. Remediation extraction methods are unviable for large contaminated areas [36-38]. Technically and financially they do not have an impressive justification for small quantities of the contaminant when it is bio-available in the soil for the plants [39]. However in mining situation, Technosol constructs and or phytostabilization, such as those proposed by Ziarati in 2014 and 2016 [40-43] may be a solution.
2.2 Consideration of plant ecological characteristics
Contaminated soil also has a large impact on the chemical composition of plants. Contaminated soil also has a large impact on the chemical composition of plants. Industrial wastewater, solid waste, municipal waste waters, and sewages from chemical factories have caused contamination of soils. The plant accessibility for particular heavy metals depends on soil pH, exchange capability and dispersal of metals between various soil fractions [41-45]. Prevalently the elements in dissolved form, with almost no suspended solids have acidic wastes combined with wastewater reaching above average COD and BOD values along with copper and zinc as heavy metal are well below the limits according to IS-3306 (1974). The total solid concentration represented in colloidal form and dissolved species in waste effluent are very likely for the fluctuation of total solid and successive dissolved solids as content collision colloidal particles [45-46]. The effectiveness of many plants in removing lead and cadmium heavy metal pollutants is a major concern affecting through soil pollution.
When investigating the comparative potential of two grasses, Panicum maximum and Axonopus compressus to bioremediate lead polluted soils, then root, shoot and leaf were analysed for morphological, biochemical parameters and Pb concentration [47]. In addition, the impact of Pb on the antioxidant defense system of the plants was studied. The root length of both P. maximum and A. compressus generally decreased as the concentration of Pb in the soil increased. At the same time the fact of reduction of the heavy metals in vegetated soils was established. However, with an insufficient length of roots, the resistance of a plant can be greatly reduced, since the plant will not receive sufficient nutrition. On the other hand, the influence of heavy metals on the genes that responsible for remediating potential of these plants remains unknown. But this is a very important question as it is necessary to avoid the extinction of these species due to the systematic use of these herbs for soil recovery from heavy metals [47].
However the phytoremediation of lead and Cadmium tendency shows that Aloe vera when grown in the soil brings appropriate hyper-accumulator by its comparatively greater ratio of biomass in the soil pollutant concentration. Aloe vera can be grown to reduce the contaminations from effluents by decreasing the heavy metal toxic levels (Shokri et al., 2016; Sawicka & Kalembasa, 2008). Physical, chemical properties and concentrations of Cadmium, Nickel and lead, as heavy metals in soils, before and after treatment of F. angulara in companion of tea determined by atomic absorption spectrophotometer. Synergic effect of mixing dried areal parts and black tea residue is found to be environmental friendly for recovering contaminated soils, and adsorb more of metal toxicity in contaminated soil [41, 45, 48]. The usual ability of a plant to volatilize a pollutant taken through roots is a natural form of air-stripping pump system. Volatile contaminants diffuse from the plant into the atmosphere through open stomata in leaves, while the radial diffusion happens through stem tissues. Microbial aromatic degradation is induced through the deposition of phenolic compounds that are basically similar to recognized inducing enzymes liable for the degradation of aromatic polluting compounds [49].
Contaminant into the Food Chain
Chemicals may have properties that can cause effects on humans and animals. Science helps protect against the potentially harmful effects of these substances by advising on safe levels of their presence in food. These levels may refer to a single / short-term high-intake of a chemical or to accumulate in the body over time. Some chemicals occur naturally in the food chain, others due to, for example, agriculture, food processing (such as the production of fries, chips) accumulate acrylamides in food and glycidyl esters – in vegetable oils and food [50-52]. Pollutants are sometimes detected in live animals and in food from animals [50]. Phytoremediation is a in situ secondary treatment methods to minimize land disturbance and eradicates transportation and liability costs related to offsite disposal management. Downward movement of pollutants arising out of percolation of rainwater can be managed with phytoremediation [38,49]. Heavy metals enter the food chain through sewage, waste generated from manufacturing activities, dust, and heavy metals in food, are common ways. Serious health issues caused by heavy metals include nervous system disorders, renal failure, genetic mutations, types of cancers, neurological disorders, respiratory disorders, and cardiovascular, immune system weakening and infertility. Milk and its products are impacted by numerous elements that are primarily involved in enzymes. The heavy metal contaminant such as lead, cadmium, chrome, nickel, and cobalt in cows though environments exposed to the livestock where the consumption of food and water are toxic affected, can disrupt milk at different levels and cause serious problems. The amount of metals in non-contaminated milk is remarkably accurate, through in vivo bio modulation but milks metal content may vary considerably through the production and packaging process [8,40,43 ,53-54]
Table 2. Pollutants demonstrated in the tested herbal drought in Poland [55].
| Herbal product |
Nitrates
[mg/100 g] |
Asphalt particles | Foreign species | Fungi of mold fungi |
Mineral impurities [mg/100 g] |
Petroleum substances [mg/100 g] |
|
1. Plantain herb: Manufacturer I Manufacturer II |
58 35 |
yes not |
not not |
not yes |
116,5 83,7 |
12-15 11-19 |
|
2. Nettle leaf: Manufacturer I Manufacturer II Manufacturer III |
91-93 170-177 111-116 |
not not not |
not not not |
not yes not |
119,4 174,3 258,9 |
18 23 51 |
|
3. knotweed herb: Manufacturer I Manufacturer II Manufacturer III |
233 117 210 |
not not not |
yes yes yes |
yes yes yes |
520,5 430,6 606,2 |
134-136 8-10 33 |
| 4. Herb of the canopy | 194-199 | Yes | yes | Not | 61,2 | 27-54 |
Contamination of commercially available herbal raw materials and their Toxicological properties
Modern and competent pharmaceutical companies and food factories in the production of herbal blends and teas comply with strict product quality standards. However, many enterprises, unfortunately, often do not comply with these strict product quality standards. The quality and the healing value of herbal products are mainly determined by the quality of the raw material (source of its acquisition) and production technology. In order to cover the ever-increasing needs of the market, professional herbal plants produce raw materials from controlled crops for their production, which on the one hand protects the natural resources of herbs, and on the other, ensures standardization of the raw material. In the case of small, unprofessional entrepreneurship and so-called common herbs in nature, the purchase of raw material from individual pickers is practiced. This is primarily about herbs referred to by botanists as ruderal and nitrous plants, such as nettle (Urtica dioica), capsella (Capsella bursa-pastoris), knotweed (Polygonum aviculare), burdock (Arctium lappa), grurz (Agropyron repens), goldenrod (Solidago virga-aurea), coltsfoot (Tussilago farfara), and comfrey (Symphytum officinale). These plants grow most, and even massively, on motorways, streets, rubbish dumps, manure, composts, railway tracks, sidewalks, roadside and drainage ditches, on debris, construction sites and gravel pits. So these are environments heavily polluted with toxic chemical
Four commonly used herbs, sold in pharmacies and herbal stores: grandmother leaf, nettle leaf, herb knotweed and herb (see Table 1) were taken for control tests. The producers of these herbs are several well-known domestic companies.
The contaminants mentioned in the table cause symptoms quite common and completely non-specific in people: gastrointestinal disorders. If the amount of oil derivatives in the portion of herbal extract taken several times exceeds 12 milligrams, diarrhea develops, nausea, headaches and dizziness, tinnitus and weakening of muscle movements. Nitrites consumed in herbs or food in the amount of 800-1000 mg act after about 30-50 minutes, redness of skin layers, headaches and dizziness, cyanosis, drop in blood pressure appear. There may be collapse, especially in the case of contaminated hypotensive herbs [8,34 ,40, 43 ,55-56].
Long-term use of herbs contaminated with these substances is manifested by anemia, granulocytopenia, proteinuria, notorious digestive disorders, drowsiness, depression and muscular weakness. Nitrites and petroleum hydrocarbons (cyclic) are carcinogenic, nephro-, neuro and hepatotoxic. In women, they cause menstrual disorders. The nature and strength of the toxic effects of herbal impurities depends on the pharmacological properties (possible interactions) of the herb. Therefore, it is necessary to strive to eliminate producers of low quality herbal products from the market by subjecting them to thorough physical, chemical and organoleptic control. The most appropriate seems be the use of plant raw materials in production from controlled crops [34,40,43, 57-59].
Contamination of the food chain with oil-derived substances
The components of petroleum, especially long-chain hydrocarbons, are very stable, non-biodegradable and remain in the environment for longer duration of time and are difficult to remove. Due to their physicochemical properties, oil-derived pollutants easily penetrate into soils, surface and ground waters, and thus into plant, animal and human organisms, causing “contamination” of all links in the food chain [60-63].
The ability of the bacteria to metabolize hydrocarbons
Bacteria (e.g. from the genus Flavobacterium, Nocardia, Mycobacterium, Pseudomonas) and fungi (e.g. Candida lipolytica yeast, Candida tropicalis) have the ability to metabolize hydrocarbons, however, rather short-chain ones. In nature, they occur near sources of oil and gas. It is possible to transfer these organisms to places contaminated with petroleum products. To this end, expensive cultures are run and produce appropriate preparations with selected cultures. However, it requires large financial outlays and technical operations [61,62].
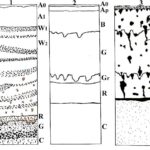
Figure 1. Soil profiles in the Wisłoka River (Poland) Valley degraded by oil-derived pollutants according to Różański [55].
Contamination of soil and water environment with crude oil
One of the anthropopressive factors is pollution of the soil and water environment with crude oil and petroleum products. This is a very serious problem due to toxicity and prevalence. Along with the development of civilization, urbanization and mechanization, the consumption of crude oil and its products increases, and the consumption of oil and environmental pollution increases. Extraction, transport, industrial processing and use of crude oil and petroleum products is associated with an ever stronger pollution of the natural environment. Drilling, use and transportation of crude oil are a source of oil-derived pollution. Crude oil initially covers plants and the topsoil. Phytocoenoses cover the earth and the intensity of atmospheric precipitation while its migration into the depth of the earth depends on the soil properties such as chemical and physical (Billet et al., 2018). During the drought spilled oil moves into the earth at a speed of about 10 cm / 45-60 min. dry soil particles, especially humus; quickly absorb oil, stopping its migration to deeper levels. In moist soil, this speed decreases by 50-60%, which is due to the hydrophobic properties of crude oil. However, as research has shown, it is the movements of the soil solution that ultimately determine the direction of migration and the distribution of oil-derived pollutants. The soil solution becomes the main carrier of oil in the soil, given that the water remains in constant motion [55].
Petroleum pollutants accumulate mainly in humus particles, as well as in soil spaces and capillaries of A0, A1, B and R levels. If the glial level G is made of dense clay and clay, then it does not let any petroleum derivatives, except for the lightest fractions and chlorofluorocarbons. If the G level contains coarse sand and stones then it becomes permeable to all hydrocarbons. Crude oil also travels along soil tumors that cut through a dense glial level. The saturation of the soil with petroleum creates anaerobic conditions and inhibits gas exchange between the atmosphere and the soil air. The soil is saturated with gaseous hydrocarbons (methane, ethane, propane, butane). Rainwater does not penetrate freely through soil levels, it stagnates. Excessive moisturizing and anaerobic environment intensifies the processes of trivalent iron reduction to divalent iron, which is manifested by sticking the soil [61, 63]. Crude oil kills small invertebrates, which diminishes the biological life of the soil. Only anaerobic bacteria live in such soil: hydrogen sulphide, hydrogen, methane and iron-reducing bacteria. Oil-degrading bacteria occur in a small amount and only in the upper levels (A0, A1), where oxygen reaches. The main function in the biodegradation of oil-derived pollutants in the oil fields is played by plants – naphthophytes [61, 64-65]. Naphthophytes are plants that have adapted well to life under extreme conditions, such as soils and waters saturated with oil or petroleum products. These plants tolerate very high concentrations of oil, eliminating (killing) other plant species. Some even clearly focus on sources of oil contamination; creating compact populations there (Table 3).
Naftophytes as a source of caries
Naftophytes are the only source of humus for these soils, and their root systems form a shallow rhizosphere. Petroleum hydrocarbons are absorbed by the roots along with the soil solution and transported by xylem to parenchyma cells. Tolerance of soil contamination level of crude oil varies depending on the species and depends on the created adaptive mechanisms and the nature of secondary metabolic pathways. The main advantages of naphthophytes are: acceleration of degradation of oil-derived pollutants. Absorption, accumulation and metabolization of hydrocarbons contained in crude oil. Oil contaminants may constitute 7 to 15% of the plant’s weight. They are humusogenic, reproduce sexually and vegetatively, grow strongly and propagate. They produce a lot of biomass. Dense root systems ensure the stability of the soil, prevent the washing out of toxic hydrocarbons and soil mass. They stop the erosion process in hilly and mountainous areas [64-67].
The cultivation of naphthophytes
The cultivation of naphthophytes on target sites is easy, fast and cheap. Care is reduced to mowing or shearing and partial harvesting of above-ground biomass. Biomass, which contains hydrocarbons inside the tissues and on the surface of the organs can be composted or processed into briquettes and used as fuel. In the case of low-grade naphthophytes, the harvested biomass harvest becomes unnecessary, it is even beneficial and purposeful to leave it in place (source of humus) [65]. The result of storing all waste is the formation of leachate due to the seepage of rainwater through the individual layers of the repository. In well-protected systems, they are collected through a system of drains and ditches and gradually discharged into tanks. The leachates are exported to the sewage treatment plant. Wastewater should meet certain standards. Meeting these requirements provides pre-treatment of leachate with a hydrophilic system. The importance of a hydrophytic pre-treatment of leachate – in the phytoremediation and biodegradation of pollutants with potential toxicity [3,5, 64,67].
Maintaining a constant qualitative and quantitative leachate chemical composition is achieved by:
- Biodegradation of toxic chemical compounds (metals, detergents, hydrocarbons, pesticides, nitrites, nitrates, phosphates, chlorides) (Barbaś & Sawicka, 2016),
- increasing the clarity of leachate,
- odorization (deodorization) of leachate,
- increasing the oxygenation of leachate,
- Humification and mineralization of organic particles,
- Induction of microfauna and microflora development responsible for the self-purification of water in nature,
- Constant biomonitoring and thus the possibility of controlling the phyto- and zootoxicity of leachates [66].
The selected plants for the pre-treatment plant should be characterized by the ability to carry out bioremediation, and at the same time a specific sensitivity to exceed toxic parameters [55, 69].
Table 3. Tolerated concentration of oil-derived pollutants in the environment [62-63].
| Species | Tolerated concentration of oil-derived pollutants in the environment (g.kg-1) | Species | Tolerated concentration of oil-derived pollutants in the environment (g.kg-1) |
| Rubus caesius L. | 230 – 306 | Lemna minor L.* | 200 – 205 |
| Achillea milefolium L. | 222 – 306 | Spirodela polyrrhiza(L.) Schleid.* | 200 – 205 |
| Digitaria sanguinalis L. | 270 – 314 |
Sparganium ramosum Huds. et S. simplex Huds.* |
204 – 210 |
| Digitaria ischaemun Schreb. | 116 – 119 | Typha latifolia L.* | 204 – 210 |
| Carex hirta L. | 470 – 504 | Polygonum amphibium L.* | 200 – 205 |
| Carex praecox Schreb. | 311 – 437 | Rumex hydrolapathum Huds.* | do 420 |
| Holcus lanatus L. | 140 – 330 | Poa annua L. | do 350 |
| Trifolium repens L. | 114 – 430 | Setaria viridis (L.) P.B. | do 320 |
| Poa pratensis L. | 112 – 320 | Taraxacum officinale Web. | do 411 |
| Linaria vulgaris L. | 170 – 420 | Phalaris arundinacea L.* | 277 – 280 |
| Juncus effusus L. | 108 – 350 | Urtica dioica L. | do 506 |
| Galium apparine L. | 107 – 330 | Carex nigra (L.) Reichard | do 411 |
| Anthoxanthum odoratum L. | 178 – 440 | Bromus hordeaceus L. | 144 – 230 |
| Salix viminalis L. | 198 – 412 | Dactylis glomerata L. | 140 – 147 |
| Salix aurita L. | to 320 | Alopecurus pratensis L. | 132 – 254 |
| Salix amygdalina L. | to 290 | Glechoma hederacea L. | ok. 260 |
| Salix repens L. | to 450 | Leontodon autumnalis L. | 102 – 256 |
| Salix purpurea L. | to 412 | Anagallis arvensis L. | do 504 |
| Salix caprea L. | to 385 | Ranunculus repens L. | do 504 |
| Prunus spinosa L. | do 412 | Polygonum hydropiper L. | 215 – 288 |
| Prunus padus L. | do 550 | Scirpus sylvaticus L.* | 303 – 309 |
| Sambucus nigra L. | do 412 | Deschampsia caespitosa(L.) P.B. | 132 – 134 |
| Rubus suberectus Weihe | 230 – 241 | Plantago maior L. | do 470 |
| Juncus inflexus L.* | 347 – 410 | Gnaphalium uliginosum L. | do 500 |
| Juncus articulatus L. | 363 – 367 | Lolium perenne L. | 132 – 166 |
| Schoenoplectus lacustris (L.) Palla* | 311 – 315 | Arrhenatherum elatius J. et C. Presl. | 200 – 300 |
| Carex hudsonii Bennet* | 403 – 407 | Equisetum palustre L. | 270 – 320 |
| Phragmites communis Trin.* | 204 – 210 |
Biological Features of Plants for Planting the Pre-treatment Plant
Water plants (hydrophytes), thanks to their own life processes, change the living conditions in the water and swamp environment, which will be shaped in the pre-treatment plant. Hydrophytes change the chemistry of water and swamp substrate by secreting its own metabolites and absorbing nutrients and toxins. Thanks to photosynthesis, they significantly increase the oxygen content in the substrate and in the water. Oxygen enables quick mineralization and biodegradation of pollutants contained in the leachates. During the night, the oxygen content decreases, and the concentration of carbon dioxide increases, which results in numerous reactions of carbonates, which detoxify (neutralize) pollutants and cause their deposition (phenols, metals) or metabolize. Hydrophytes absorb salts of calcium, lead, phosphates, nitrates and nitrites, ammonium compounds from the effluents [67, 70-71] : Dead plant remains undergo humification and form a humus substrate (sludge), constituting the living environment of microorganisms (bacteria, protozoa, cyanobacteria, algae) which gives rise to a well-functioning ecosystem that is necessary for leaching. It is necessary to focus on such features:
- Root systems and humus deposits are a natural biofilter that retains and degrades the effluent contaminants. Airing the sediment-biofilter provides not only oxygen from photosynthesis;
- the air reaches the deeper levels through the aeration tissues of the plants contained in the shoots, rhizomes and roots, and which constantly breathe;
- dead plant organs also constitute the reservoir of air (aerenchyma, slowly dying, but filled with air) [61, 67,69]. To endure through the prolonged hypoxic environments, water plants obligate to established adaptations and contrivances at the essential level. In the case of roots, the most common morphological and functional modifications contain the creation of accidental roots, aerenchyma and hypertrophic lenses. These alterations advance the ability to capture oxygen being transported to the immersed tissues of the plants [67,69]. Libertia grandiflora has two types of roots one that adsorb nutrients and ascribe to the plant in the soil, and the other an accidental roots that are along the shoots and provide oxygen uptake [68,72]. In addition there are many semi and fully aquatic species which have established a large number of aerenchyma in roots, stems and leaves [61, 70,73]. Ecological and morphological types of plants used in the hydrophilic system;
- Plants with floating leaves or shoots partially protruding from the water. Species completely submerged, floating on the surface of the water and partially emerged;
- Air-water plants, rooted in the bottom of the reservoir. Assimilation shoots exist above the surface of the water;
Aquatic plants, growing near water reservoirs, which can be periodically flooded [61,66, 70, 72,74].
Discussion
In various regions of the world, plant species have been identified that are capable of absorbing heavy metals and restoring contaminated soil. However, the potential of these plants and their stability is uneven.
As shown by multiple studies, many plant species most easily absorb cadmium and lead, which are particularly toxic in relatively low dosages. This applies to any plants, even those that grow spontaneously. In this regard, plants that grow on soil contaminated by cadmium, are secondary sources of environmental hazards. This is because these plants deliver heavy metals to living organisms along the food chain. Consequently, it is especially unacceptable to cultivate commercial raw materials on lands contaminated by cadmium and lead. Such pollution is characterized, for example, by the roadsides of highways, where often collect medicinal herbs.
At the same time, cadmium and lead are toxic directly to plants that ingest these metals. Studies have shown that when a plant grows on soil contaminated with lead, the length of the roots decreases as the concentration of lead in the soil increases. This indicates that the amount of nutrients for the plant will decrease, and they can die off sooner than when grown on clean soil. It should be noted that a promising plant in phytoremediation technologies is Aloe vera when grown in soil contaminated with lead and cadmium. According to research, this plant manifests itself as a hyper-accumulator. It is also very important that Aloe vera is widely cultivated throughout the world. Also, this plant can be grown to reduce pollution from wastewater by reducing the level of toxicity of heavy metals.
Special types of pollution include oil pollution. For their removal, naphtofites are promising, which are the only source of humus for these soils.
Also, the effect of heavy metals on plant genes responsible for the restoration of their potential remains unknown. But this is a very important issue, since it is necessary to avoid the extinction of certain plant species due to their systematic use for the recovery of soil from heavy metals.
Conclusions
1. Prospects for using soil restoration for heavy metals are very limited. One of the limitations is a high degree of soil contamination. The second limitation is the high probability of contaminants entering the body of animals or people.
2. To eliminate the first restriction, it is necessary to study more deeply the effects of heavy metals on plant genes that are used for phytoremediation. The questions of the influence of heavy metals on plant genes deserve serious attention. This is important to prevent reduce species diversity in nature. However, today these issues are very little studied.
3. To eliminate the second limitation, timely removal of spent plants is required. As the literature review and results of this study show, each plant species has its own limit for the absorption of heavy metals (that is, each plant species has different phytoremediation effectiveness). Thus, at the moment when the highest efficiency of absorption of heavy metals is achieved, the plants should be removed and new planted for further soil cleaning, if necessary.
Conflict of interest
None of the authors have any conflicts of interest associated with this study.
Acknowledgments
The authors are thankful to administration of Nutrition and Food Sciences Research center, Tehran Medical Sciences, Islamic Azad University (Tehran-Iran) for their support, as well as the administrations of all other universities in which the authors of this article work. This research is not funded by a specific project grant.
References
1. Tarczewska A, Kozłowska M, Dobryszycki P, Kaus-Drobek M, Dadlez M , Ożyhar A.Insight into the Unfolding Properties of Chd64, a Small, Single Domain Protein with a Globular Core and Disordered Tails. PLoS ONE. 2015; 10(9): e0137074.
2. Sawicka B , Kalembasa D. Annual variability of some toxic element contens (Cd, Cr, Co, Ni and Pb) and response of two Jerusalem artichoke varieties to increasing nitrogen fertilizer at constant PK levels. Pol. J. Environ. Stud. 2013; 22(3): 861-71.
3. Ahmadi M, Ziarati P, Manshadi M, Asgarpanah J, Mousavi Z. The Phytoremediation Technique for Cleaning Up Contaminated Soil by Geranium (Pelargonium roseum). Intl. J. Farm. & Alli. Sci. 2013; 15: 477-81.
4. Ziarati P, Ziarati NN, Nazeri S, Saber-Germi M. Phytoextraction of Heavy Metals by two Sorghum Spices in Treated Soil Using Black Tea Residue for Cleaning-Up the Contaminated Soil. Orient J. Chem. 2015; 31(1): 317-26.
5. Manshadi M, Ziarati P, Ahmadi M, Fekri K. Greenhouse Study of Cadmium and Lead phytoextraction by five Pelargonium spices. Intl. J. Farm & Alli. Sci. 2013;2 (18): 665-9.
6. Shokri F, Ziarati P , Mousavi Z.Removal of Selected Heavy Metals from Pharmaceutical Effluent by Aloe Vera L. Biomedical Pharmacol J. ., 2016; 9(2): 705-13.
7. Ziarati P, Asgarpanah J, MirMohammad Makki F. Phytoremediation of Heavy Metal Contaminated Water Using Potential Caspian Sea Wetland Plant: Nymphaeaceae. Biosci. Biotech. Res. Asia. 2015; 12(3): 2467-73.
8. Ziarati P, Shirkhan E, Mostafidi M, Zahedi M,T. An Overview of the Heavy Metal Contamination in Milk and Dairy Products. Acta Scientific Pharmaceutical Sciences 2018;2(7): 1-14.
9. Vambol V. Numerical integration of the process of cooling gas formed by thermal recycling of waste. Eastern-European Journal of Enterprise Technologies. 2016; 8(84): 48–53.
10. Koloskov V. Vyznachennya znachushchykh pokaznykiv kryteriyu ekolohichnoho rezervu terytoriy, prylehlykh do mists zberihannya vidkhodiv. Technogenic and ecological safety. 2018, 3(1): 44–51. (doi: 10.5281/zenodo.1182841).
11. Vambol S, Koloskov V, Derkach Yu. Assessment of environmental condition of territories adjoined to wastes storage sites based on environmental reserve criterion. Technogenic and ecological safety. 2017; 2: 67–72. (http://nbuv.gov.ua/UJRN/techecolsaf_2017_2_12).
12. Vambol S, Vambol V, Bogdanov I, Suchikova Y , Rashkevich N. Research of the influence of decomposition of wastes of polymers with nano inclusions on the atmosphere. Eastern-European Journal of Enterprise Technologies . 2017; 10(90): 57–64. (doi: 10.15587/1729-4061.2017.118213).
13. Kondratenko OM, Vambol SO, Strokov OP, Avramenko AM. Mathematical model of the efficiency of diesel particulate matter filter. Naukovyi Visnyk Natsionalnoho Hirnychoho Universytetu. 2015; 6(150): 55–61.
14. Balaceanu CM, Iordache G. Assessment of the air pollution at the industrial stations in metropolitan area of Bucharest. Naukovo-tekhnichnyy zhurnal “Tekhnohenno-ekolohichna bezpeka” . 2018, 3(1) : 8–15.
15. Vambol S, Vambol V, Kondratenko O, Suchikova Y, Hurenko O. Assessment of improvement of ecological safety of power plants by arranging the system of pollutant neutralization. Eastern-European Journal of Enterprise Technologies. 2017; 10(7): 63–73.
16. Kolesnyk VY, Pavlychenko AV, Buchavyy YV. Unifikovana metodyka kompleksnoho otsinyuvannya rivnya ekolohichnoyi nebezpeky promyslovykh obyektiv ta tekhnolohiy. Naukovo-tekhnichnyy zhurnal “Tekhnohenno-ekolohichna bezpeka” 2018; 3(1): 64–9.
17. Vambol S, Kondratenko O. Results of complex criterial fuel and ecological assessment of diesel engine 2Ch10.5/12 for emergency and rescue power plants. Naukovo-tekhnichnyy zhurnal “Tekhnohenno-ekolohichna bezpeka”. 2017; 1: 32–8.
18. Vambol S, Shakhov Y, Vambol V, Petukhov I. A mathematical description of the separation of gas mixtures generated by the thermal utilization of waste. Eastern-European Journal of Enterprise Technologies. 2016; 2(79): 35–41.
19. Tyutyunyk VV, Strilets VM, Kaluhin VD, Zakharchenko YV. Rozvytok metodolohichnoho pidkhodu dlya tekhnohenno-ekolohichnoyi otsinky rivnya nebezpeky funktsionuvannya lokalnykh terytoriy Ukrayiny. Tekhnohenno-ekolohichna bezpeka. 2018, 3(1): 91-101.
20. Popov O, Іatsyshyn A, Kovach V, Artemchuk V, Taraduda D, Sobyna V, Sokolov D, Dement M, Yatsyshyn T. Conceptual Approaches for Development of Informational and Analytical Expert System for Assessing the NPP impact on the Environment. Nuclear and Radiation Safety. 2018; 3(79): 56-65.
21. Vambol S, Vambol V, Kondratenko O, Koloskov V, Suchikova Y. Substantiation of expedience of application of high-temperature utilization of used tires for liquefied methane production, Archives of Materials Science and Engineering. 2018; 2(87): 77-84. (doi: 10.5604/01.3001.0012.2830).
22. Barsukova G. Development of mathematical model of infiltration of iron sulfate acid solution. Technogenic and ecological safety. 2018, 4(2): 99–104.
23. Sihag P , Singh B. Field evaluation of infiltration models. Technogenic and ecological safety. 2018, 4(1): 3–12. (doi: 10.5281/zenodo.1239447).
24. Zariati P, Namvar S, Sawicka B. Heavy metals bio-adsorption by Hibiscus sabdariffa L. from contaminated water. Technogenic and Ecological Safety. Scientific and Technical Journal. 2018; 4(2): 22-32.
25. Sova AH, Jakabova S, Simon L, Heged OS, Vargova A, Talos K, Pernyes T, Majdik C. Induced phytoextraction of lead from contaminated soil. Acta Universitatis Sapientiae Agriculture and Environment. 2009; 1: 116-22.
26. El Din Mahmoud A, Fawzy M. Bio-based Methods for Wastewater Treatment: Green Sorbents, [in:] A.A. Ansari, S.S. Gill, R. Gill, G.R. Lanza, L. Newman (eds), Phytoremediation: Management of Environmental Contaminants, 3rd edition. Springer International Publishing, Cham, Switzerland: 2016; 209-38.
27. Smolders E. Cadmium uptake by plants (paper presented at the conference – Metal in Eastern and Central Europe: Health effects, sources of contamination and methods of remediation 2001). International Journal of Occupational Medicine and Environmental Health. 2001; 14(2): 177-83.
28. Ziarati P, Alaedini S. The Phytoremediation Technique for Cleaning up Contaminated Soil By Amaranthus sp. J. Environ. Anal. Toxicol. 2014; 4(2): 208. (doi: 10.4172/2161-0525.1000208).
29. Sawicka B, Pszczółkowski P. Pollution of tubers of early potato varieties with lead and strontium in Eastern Polesie, [in:] Effects on organisms and environment, III Conference on Trace Metals, 6-8 September.2000, Sopot/Poland, Book of abstracts. Institute of Oceanography PAS, University of Gdańsk, Sopot: 2000; 235.
30. Tavakoli-Hosseinabady B, Ziarati P, Ballali E, Umachandran K. Detoxification of Heavy Metals from Leafy Edible Vegetables by Agricultural Waste: Apricot Pit Shell. J Environ Anal Toxicol. 2018; 8: 548. doi: 10.4172/2161-0525.1000548.
31. Małachowska-Jutsz A. Mikoryzacja roślin a efektywność fitoremediacji gruntów zanieczyszczonych węglowodorami / Plant micorrhization versus effectiveness of phytoremediation of soil polluted with hydrocarbons. Zeszyty Naukowe. Inżynieria Środowiska / Politechnika Śląska. 2008; 59: 1-161.
32. Kokyo O, Tiehua C, Tao L, Hongyan C. Study on Application of Phytoremediation Technology in Management and Remediation of Contaminated Soils. Journal of Clean Energy Technologies. 2014; 2(3): 216-20.
33. Mahmoud AED, Fawzy M, Radwan A. Optimization of Cadmium (CD2+) removal from aqueous solutions by novel biosorbent. International Journal of Phytoremediation. 2016; 18(6): 619-25.
34. Nouri J, Khorasani N, Lorestani N, Karami M, Hassani AH, Yousef N. Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ. Earth Sci. 2009; 59: 315–23.
35. Moniczewski A, Starek M, Rutkowska A. Toxicological aspects of metal impurities in cosmetics (Toksykologiczne aspekty zanieczyszczeń metalicznych w kosmetykach). 2016; 2 (107): 81-9.
36.Ziarati P, Nazif MH. , Khandehrouy M. Decreasing Bio-toxicity of Fume Particles Produced in Welding Process by Aloe Vera L. Oriental J. Chem. 2015; 31(Special Issue): 113-20.
37.Baccio D, Tognetti R, Sebastiani L, Vitagliano C. Responses of Populus deltoides× Populus nigra (Populus× americana) clone I214 to high zinc concentrations. New Phytologist. 2003; 159(2):443-52.
38.Ziarati P , Alimardan M. Study on Increasing Efficiency of Phytoremediation in Cadmium and Nickel Contaminated Soil. Chemistry Journal. 2015; 5(6): 86-92.
39.Rajakaruna N, Tompkins KM, Pavicevic PG. Phytoremediation: An affordable green technology for the clean-up of metal contaminated sites in Sri Lanka. Ceylon J. Sci. (Biological Sciences). 2006; 35: 25-39.
40.Ziarati P, Hosseini A. The effect of fennel (Foeniculum vulgare) on potential phytoremediation of Pelargonium. International Journal of Plant, Animal and Environmental Sciences. 2014; 4 (3): 438- 44.
41.Jalillian Z, Ziarati P. High potential of Ferulago Angulate (Schecht) Boiss in Adsorption of Heavy Metals. Biomed. Pharmacol. J. 2016;9(1): 201-8.
42.Ziarati P, Zolfaghari M, Azadi B. The effect of Tea Residue in Promoting Phytoremediation of Lavendula angustifoli Mill. International Journal of Plant, Animal and Environmental Sciences. 2014; (4) 2: 479-86.
43.Ziarati P, Iranzad-Asl S, Asgarpanah J. Companion Pelargonium roseum and Rosmarinus officinalis in cleaning up contaminated soil by phytoextraction technique: the role of companion plants in boosting phytoremediation potential. International Journal of Plant, Animal and Environmental Sciences. 2014; 4(3): 424-30.
44.Sawicka B. Cadmium and nickel levels in tubers of Helianthus tuberosus L., [in:] Effects on organisms and environment, III Conference on Trace Metals, 6-8 September.2000, Sopot/Poland, Book of abstracts. Institute of Oceanography PAS, University of Gdańsk, Sopot: 2000; 98.
45.Sawicka B, Kalembasa D. Variability in macroelement content in tubers of Helianthus tuberosus L. at different nitrogen fertilization levels. Acta Sci. Pol. Agricultura. 2008; 7(1): 67-82.
46.Pourzare A, Ziarati P, Mousavi Z, Faraji AR. Removing Cadmium and Nickel Contents in Basil Cultivated in Pharmaceutical Effluent by chamomile (Matricaria chamomilla L.) Tea Residue. J. Sci. Discov. 2017; 1(1): jsd17006
47.Ukoh SNB, Akinola MO, Njoku KL. Comparative study on the growth response and remediation potential of Panicum maximum and Axonopus compressus in lead contaminated soil. Technogenic and ecological safety. 2019; 5(1): 3–12.
48.Danilčenko H, Jarienė E, Gajewski M, Cerniauskiene J, Kulaitiene J, Sawicka B, Aleknaviciene P. Accumulation of elements in some organically grown alternative horticultural crops in Lithuania. Acta Sci. Pol. Hortorum Cultus. 2011; 10(2): 23-31.
49.Kamath R, Rentz JA, Schnoor JL , Alvarez PJJ. Phytoremediation of hydrocarbon-contaminated soils: principles and applications. Pet. Biotechnol. Dev. Perspect. 2004; 151: 447–78.
50.Sawicka B, Mohammed A, Umachandran K. Food safety of potato processed in the aspect of acrylamide risk. MOJ Food Process Technol. 2018; 6(3): 96-102.
51.Umachandran K, Sawicka B, Abdul-Nabi NN , Pasqualone N. Nutritional features of Biryani as the basis for the formation of an entrepreneurial mode in Biryani Market. Journal Advances in Agricultura. 2018; 8(1): 1268-78.
52.Umachandran K, Sawicka B, Mohammed A, Nasir NN , Pasqualone A. Relevance of nanotechnology in food processing industries. International Journal of Agriculture Sciences. 2018; 10(7): 5730-3.
53.Kotiuk E , Sawicka B. Evaluation of Heavy Metals Content in Filled Wafers with Vitamin and UFA n-3 Addition. Polish J. Environ. Stud. 2008; 17(1): 243-8.
54.Sawicka B, Noaema AH, Hameed ST, Skiba D. Genotype and environmental variability of chemical elements in potato tubers. Acta Sci. Pol. Agricultura. 2016; 15(3): 79-91.
55.Różański M. Bezpieczeństwo stosowania produktów pochodzenia naturalnego w Europie i Ameryce Północnej. Badanie ankietowe dotyczące stosowania produktów pochodzenia naturalnego w Polsce. Praca doktorska, Katedra i Zakład Farmakologii Doświadczalnej i Klinicznej Warszawskiego Uniwersytetu Medycznego [Safety of using natural products in Europe and North America. Survey on the use of natural products in Poland]. PhD Thesis, Department of Experimental and Clinical Pharmacology of the Medical University of Warsaw], Warszawa. 2012.
56.Sawicka B, Barbaś P. Macroelements Variability in the Potato Tubers under Organic and Integrated Crop Production System. Polish Journal of Environmental Studies. 2007; 16(3A): 227-30.
57.EN ISO 6888-2, 1999, Microbiology of food and feed – horizontal determination method the number of coagula-positive staphylococci.
58.Obawede K, Umoh J. Contamination of Infants Powdered milk in use with enterotoxigenic Staphylococcus aureus. Food Microbiol. 2004; 2: 255-61.
59.De Blackburn CW, McClure PJ. Foodborn pathogens. Hazards, risk analysis and control. Woodhead Publishing Limited, Camridge England: 2002;385-90.
60.Billet K, Genitoni J, Bozec M, Renault D, Barloy D. Water and land morphotypes of the invasive water plant, Ludwigia grandiflora, show distinct morphological and metabolic reactions. Ecol. Evol. 2018; 8(5): 2568-79.
61.Gillard M, Thiébaut G, Deleu C, Leroy B. Current and future distribution of three taxa of aquatic plants around the world: Reduction in native numbers and increase of invasive coverage. Biological Invasions . 2017;19: 2159-70.
62.Różański H, Właściwości toksykologiczne wód zanieczyszczonych węglowodorami fluoro- i chloropochodnymi. IV Krajowa Konferencja Polskiego Towarzystwa Medycyny Środowiskowej [Toxicological properties of waters contaminated with fluoro- and chlorinated hydrocarbons. IV National Conference of the Polish Society of Environmental Medicine], Ustroń-Jaszowiec. 2017.
63.Różański H. Oddziaływanie zanieczyszczeń ropopochodnych na gleby. Ekotoksykologia, medycyna środowiskowa i fitofarmakologia. 2001. Available on site : www.luskiewnik.gower.pl/PRYSZCZE2002.htm.
64.Różański H. Vegetation of oil fields in the Krosno Province. Typescript. Research sponsor: the Büchner Foundation, Krosno-Poznań: 1997; 12-;4.
65.Różański H. Chemical composition and ecotoxicological properties of wastewater from kerosene and gas mines. II National Conference of the Polish Society of Environmental Medicine. Mat. Conf. Environmental Medicine, Warężyno; 1999; 53-5.
66.Gladish DK, Xu J, Niki T. Programmed death programmed in a similar way to apoptosis occurs in humus and ground pea meristem (Pisum sativum) exposed to sudden floods. Annals of Botany . 2006; 97: 895-902.
67.Gladish DK, Xu J, Niki T. Programmed death programmed in a similar way to apoptosis occurs in humus and ground pea meristem (Pisum sativum) exposed to sudden floods. Annals of Botany. 2006; 97: 895-902.
68.EPPO. Ludwigia grandiflora i L. peploides, Onagraceae – Pierwiosnki wodne [Ludwigia grandiflora and L. peploides, Onagraceae – Water primroses]. Bulletin EPPO. 2011; 41: 414-8.
69.Malik AI, Colmer TD, Lambers H , Schortemeyer M. Aerenchyma formation and radial O2 loss along the side-wheat roots only the apical part of the main exposed to O2 deficiency. Plant, Cell & Environment. 2003; 26(10): 1713-22.
70.Nowak JS, Ono J, Cronk QCB. Anatomical examination of water mustard: Subularia aquatica (Brassicaceae). Aquatic Botany. 2010; 93: 55-8.
71.Barbaś P, Sawicka B. Impact of weeding methods on the content of nitrates in potato tubers. Nauka Przyr. Technol. 2016; 10(4): 49.
72.Li Z, Yu D , Xu J. Adaptation to water level variation: Responses of a floating-leaved macrophyte Nymphoides peltata to terrestrial habitats. Annales de Limnologie-International Journal of Limnology. 2011; 47(01): 97-102.
73.Mahmoud AED, Fawzy M, Radwan A. Optimization of Cadmium (CD2+) removal from aqueous solutions by novel biosorbent. International Journal of Phytoremediation. 2016; 18(6): 619-25.
74.Sawicka B, Kotiuk E. Evaluation of health safety of mustards in the obligatory norms. Acta Sci. Pol. Technol. Alim. 2006; 5(2): 165-77.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/