J Med Discov (2019); 4(1):jmd19001; DOI:10.24262/jmd.4.1.19001; Received January 09th, 2019, Revised March 04th, 2019, Accepted March 16th, 2019, Published March 22nd, 2019.
Intrarenal Dopamine Metabolism
Isaac D. Hodapp, He Liu
Biology Department, Morosky College of Health Professions and Sciences, Gannon University, Erie, PA, USA.
* Correspondence:He Liu, Email: liu017@gannon.edu
Abstract
In the past 40 years, dopamine has been shown to have a greater influence on the body than previously thought. Not only is it produced in the central nervous system as a neurotransmitter, but also by the renal system regulating sodium homeostasis and influencing vasodilation. The metabolism of dopamine in the renal system starts with L-DOPA uptake, which is thought to be the rate-limiting step of dopamine synthesis, performed by Na+-dependent cotransporters (B0 and ASCT2) and Na+-independent transporters (LAT1 and LAT2). Variations as to which transporter is dominant can be observed in hypertensive vs. normal animals. L-DOPA uptake is affected by Na+ concentrations, gastrin concentration, and activation of α(2C)-adrenoceptor. The synthesis of dopamine is through the decarboxylation of L-DOPA catalyzed by aromatic L-amino acid decarboxylase and is influenced by Na+ concentration (where greater Na+ molarity increases activity). The degradation of dopamine is completed by monoamine oxidase and catechol-O-methyltransferase before it is excreted into the urine as homovanillic acid. While this process shows the complete metabolism of dopamine, there are other findings that suggest alternative pathways of dopamine production. This review will provide greater details regarding the metabolism steps of dopamine in the renal system.
Keywords: dopamine, kidney, L-DOPA, sodium, blood pressure
Introduction
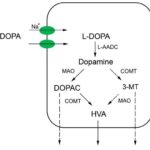
Within the past 40 years dopamine, a catecholamine which is a precursor for epinephrine and norepinephrine, an important neurotransmitter in the brain, has been seen to hold an even greater array of influence on the body than the many actions previously thought: efficient movement, pleasure driven behavior, delicate equilibrium for optimal memory, directing attention, problem solving, prolactin secretion, pain control, and nausea control [1-3]. It wasn’t until dopamine infusions were found to improve kidney function in acute renal failure patients and urinary concentration of dopamine were noted as being up to 20 times greater than the concentration of noradrenaline that the possibility of dopamine having an even wider spectrum of influences on the body arose [4]. Dopamine receptors have been apparent in various peripheral tissues such as the kidneys, lungs, blood vessels and pancreas [5]. The only place noted for dopamine production outside of the central nervous system has been the renal system. Dopamine is shown to have a rather large influence on sodium homeostasis by controlling excretion as well as holding vasodilator properties, both of which help to maintain a normal blood pressure [6]. The physiological effects of dopamine may attribute to both the metabolism to maintain the dopamine level and the variation in dopamine receptors. This review, based on related research articles in PubMed, will provide greater details regarding the dopamine metabolism in the renal system (Fig. 1), including the processes of L-DOPA uptake and what influences it, decarboxylation of L-DOPA into dopamine, degradation of dopamine until excretion, and lastly evidence that suggests alternative pathways.
L-DOPA Uptake
The uptake of L-DOPA is regarded as being the rate-limiting step of dopamine synthesis [7]. The substrate of dopamine, L-dihydroxyphenylalanine (L-DOPA), is transported into the proximal renal tubule epithelial cells through several carrier proteins, including both Na+-dependent cotransporters and Na+-independent transporters. L‑type amino acid transporter 1 (LAT1) and L‑type amino acid transporter 2 (LAT2) are responsible for L-DOPA uptake in a Na+ independent manner [7-10]. In wild-type rats, LAT2 was found almost exclusively responsible for L-DOPA uptake [7, 11]. However, in Na+-free medium, human kidney slides only accumulated nearly half of the amount of dopamine found at 160 mM Na+ as shown in an earlier study [12], presumably via LAT2. In pig renal epithelial cells, gene silencing of LAT2 with siRNA reduced L-DOPA accumulation by 85% [13, 14]. Some studies showed that the accumulation of L-DOPA in the epithelial cells was dependent on extracellular Na+ [15, 16]. Inhibition of the enzyme Na+/K+ ATPase by ouabain in high Na+ medium reduced the concentration gradient across the cell membrane and levels of dopamine and its metabolites in the cell [12, 15]. Na+-dependent amino acid transport systems, in particular, B0 and ASCT2, seemed involved in the L-DOPA uptake [7-10, 17]. In the spontaneously hypertensive rat model, L-DOPA uptake was mediated by multiple carriers (50% through LAT1, 25% through LAT2, and 25% through Na+-dependent amino acid transport system B0 [7]). The involvement of Na+-dependent transporters makes the L-DOPA uptake responsive to extracellular Na+ concentration. In cultured opossum kidney cells, low sodium solution (137 mM K+ plus 5 mM Na+) reduced L-DOPA uptake by 25% compared to the control condition (137 mM Na+ plus 5 mM K+) [18]. In animal experiments, the responses appeared to be age-dependent and with a variation in the regulation pattern of transporter gene expressions. Table 1 lists the results of these studies.
The uptake of L-DOPA is under the effect of other hormones. Gastrin concentration increase from 25 nM to 50 nM stimulated the renal tubular uptake of L-DOPA by almost two-fold via LAT1 in cultured human and mouse cells [22]. In contrast, the activation of α(2C)-adrenoceptor inhibited L-DOPA uptake [23].
Decarboxylation of L-DOPA
After uptake into the cytoplasm, L-DOPA is converted into dopamine through a decarboxylation reaction catalyzed by aromatic L-amino acid decarboxylase (L-AADC or AADC), which was found at a high level in mouse kidney (both cortex and medulla) [24] and in human kidney homogenate [25]. In particular, L-AADC was found only in the proximal convoluted tubule and proximal straight tubules of the rat nephron [26]. High-salt intake increased L-AADC activity in rats, while there was no significant change in dopamine degradation [27-29]. Carbidopa, a peripheral DOPA-decarboxylase inhibitor significantly decreased urinary dopamine output and sodium excretion in humans [30-32].
Fig 1. Cellular pathway of dopamine metabolism in proximal renal tubule epithelial cells. L-DOPA: L-dihydroxyphenylalanine; DOPAC: dihydroxyphenylacetic acid; 3-MT: 3-methoxytyramine; HVA: homovanillic acid; L-AADC: L-amino acid decarboxylase; MAO: monoamine oxidase; COMT: catechol-O-methyltransferase. Solid lines denote main pathways while dashed lines denote alternative pathways.
Degradation
As shown in Figure 1, dopamine is catabolized by the enzyme monoamine oxidase (MAO) into dihydroxyphenylacetic acid (DOPAC), then further methylated by another enzyme catechol-O- methyltransferase (COMT) to its final metabolite homovanillic acid (HVA) before being excreted in the urine [33]. The combination of the two enzymes in the reverse order degrades dopamine in an alternative pathway to the same product [33]. Administration of COMT inhibitors entacapone [34, 35], nitecapone [36], or 3,5-dinitrocatechol [37] elevated renal dopamine activity and increased sodium excretion in a more effective manner than L-DOPA [35] as well as the kidney-specific pro-drug to dopamine, glu-dopa [36]. In mice with one or both copies of the COMT gene deleted, the natriuretic effects of acute sodium loading were reduced by 60-70% [9]. Administration of the MAO inhibitor phenelzine also showed similar effects [36].
Table 1. Age-dependent and strain-dependent variations in the regulation of L-DOPA transporter gene expressions under high-salt intake
| Strain | Age Comparison | Effects | Gene Expression | Reference | |
| Young | Old | ||||
| Fischer 344 rats | 6-month | 24-month |
Age effect: 2-fold increase in urinary dopamine and DOPAC, but not seen in old rats.
High-salt effect: 2.6-fold increase in natriuretic response seen in young rats but only 2.3-fold in old rats.
|
N/A | [19] |
| Wistar-Kyoto rats | 4-week | 12-week |
High-salt effects: higher urinary dopamine and DOPAC in young rats, but no change in old rats.
|
High-salt effects: higher LAT1, lower LAT2, lower B0and lower ASCT2 in young and rats; lower LAT1, lower LAT2, lower B0 and lower ASCT2 in old rats. Age effects: higher LAT1, LAT2, lower ASCT2, and lower lower B0 in old rats.
|
[17, 20] |
| Spontaneously hypertensive rats (SHR) | 4-week | 12-week |
High-salt effects: higher dopamine and DOPAC in both young and old rats. Higher tubular update of L-DOPA.
|
High-salt effects: lower LAT1, lower LAT2, and lower B0 in young rats; lower LAT2, higher ASCT2, and higher B0 in old rats.
|
[17, 20] |
| Wistar-Kyoto rats | 13-week | 91-week |
Age effects: no significant difference in urinary L-DOPA, dopamine, and DOPAC between young and old rats.
|
Age effects: lower LAT1, higher LAT2, higher ASCT2 expression in old rats. |
[21] |
| Spontaneously hypertensive rats (SHR) | 13-week | 91-week |
Age effects: higher urinary L-DOPA in young rats but higher urinary DOPAC in old rats.
|
Age effects: lower LAT1, higher LAT2, and higher ASCT2 expression in old rats. |
[21] |
Decarboxylation of L-DOPA
After uptake into the cytoplasm, L-DOPA is converted into dopamine through a decarboxylation reaction catalyzed by aromatic L-amino acid decarboxylase (L-AADC or AADC), which was found at a high level in mouse kidney (both cortex and medulla) [24] and in human kidney homogenate [25]. In particular, L-AADC was found only in the proximal convoluted tubule and proximal straight tubules of the rat nephron [26]. High-salt intake increased L-AADC activity in rats, while there was no significant change in dopamine degradation [27-29]. Carbidopa, a peripheral DOPA-decarboxylase inhibitor significantly decreased urinary dopamine output and sodium excretion in humans [30-32].
Degradation
As shown in Figure 1, dopamine is catabolized by the enzyme monoamine oxidase (MAO) into dihydroxyphenylacetic acid (DOPAC), then further methylated by another enzyme catechol-O- methyltransferase (COMT) to its final metabolite homovanillic acid (HVA) before being excreted in the urine [33]. The combination of the two enzymes in the reverse order degrades dopamine in an alternative pathway to the same product [33]. Administration of COMT inhibitors entacapone [34, 35], nitecapone [36], or 3,5-dinitrocatechol [37] elevated renal dopamine activity and increased sodium excretion in a more effective manner than L-DOPA [35] as well as the kidney-specific pro-drug to dopamine, glu-dopa [36]. In mice with one or both copies of the COMT gene deleted, the natriuretic effects of acute sodium loading were reduced by 60-70% [9]. Administration of the MAO inhibitor phenelzine also showed similar effects [36].
Alternative pathways
In addition to the pathways described above, it is possible that other unknown pathways are present. An earlier study showed that homogenized kidney tissue increased dopamine production under elevated NaCl, KCl, and NH4Cl [38]. A higher intake of phosphate was observed to increase dopamine excretion [39]. Lastly, 3-O-methyldopa (OM-dopa) has been found to be a substrate for intrarenal dopamine production through demethylation [40].
Conclusion
Dopamine plays a key role in sodium homeostasis and vasodilation, both of which contribute to blood pressure regulation. Understanding the metabolism of dopamine in the renal system can lead us toward better treatment options for disorders such as kidney failure and hypertension. The rate of dopamine synthesis is dependent on L-DOPA uptake by Na+-dependent cotransporters (B0 and ASCT2) and Na+-independent transporters (LAT1 and LAT2). Observations in hypertensive vs. normal animals showed variations in the dominant roles of different transporters. The rate of uptake is influenced by a few factors including Na+ concentration, gastrin concentration, and activation of α(2C)-adrenoceptor. Na+ concentration also holds influence on the decarboxylation of L-DOPA which is catalyzed by L-AADC. Dopamine may be degraded then excreted in the urine as HVA after being broken down by MAO and COMT. Dopamine synthesis may also have other pathways indicated by another possible substrate 3-O-methyldopa and the influence of NaCl, KCl, NH4Cl, and phosphate on renal dopamine production.
Conflict of interest
None
Acknowledgments
None (no grant support of this work).
References
1. Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol Neurobiol 2018.
2. Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol 2002; 67(1): 53-83.
3. Nieoullon A, Coquerel A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol 2003; 16 Suppl 2S3-9.
4. Lee MR. Dopamine and the kidney. Clin Sci (Lond) 1982; 62(5): 439-48.
5. Drozak J, Bryla J. Dopamine: not just a neurotransmitter. Postepy Hig Med Dosw (Online) 2005; 59405-20.
6. Romero-Vecchione E, Vasquez J, Velasco M, Morales E, Davoli MA, Arocha I. Increase of renal dopamine production induced by nifedipine in hypertensive patients. Double blind vs placebo study. Arch Inst Cardiol Mex 1995; 65(6): 535-40.
7. Pinho MJ, Serrao MP, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Over-expression of renal LAT1 and LAT2 and enhanced L-DOPA uptake in SHR immortalized renal proximal tubular cells. Kidney Int 2004; 66(1): 216-26.
8. Gomes P, Soares-da-Silva P. Na+-independent transporters, LAT-2 and b0,+, exchange L-DOPA with neutral and basic amino acids in two clonal renal cell lines. J Membr Biol 2002; 186(2): 63-80.
9. Quinones H, Collazo R, Moe OW. The dopamine precursor L-dihydroxyphenylalanine is transported by the amino acid transporters rBAT and LAT2 in renal cortex. Am J Physiol Renal Physiol 2004; 287(1): F74-80.
10. Pinto V, Pinho MJ, Soares-da-Silva P. Renal amino acid transport systems and essential hypertension. FASEB J 2013; 27(8): 2927-38.
11. Wu Y, Yin Q, Lin S et al. Increased SLC7A8 expression mediates L-DOPA uptake by renal tubular epithelial cells. Mol Med Rep 2017; 16(1): 887-93.
12. Soares-da-Silva P, Pestana M, Fernandes MH. Involvement of tubular sodium in the formation of dopamine in the human renal cortex. J Am Soc Nephrol 1993; 3(9): 1591-9.
13. Soares-Da-Silva P, Serrao MP, Pinho MJ, Bonifacio MJ. Cloning and gene silencing of LAT2, the L-3,4-dihydroxyphenylalanine (L-DOPA) transporter, in pig renal LLC-PK1 epithelial cells. FASEB J 2004; 18(13): 1489-98.
14. Soares-da-Silva P, Serrao MP. Apical and basolateral 4F2hc and the amino acid exchange of L-DOPA in renal LLC-PK1 cells. Amino Acids 2005; 29(3): 213-9.
15. Soares-da-Silva P, Fernandes MH, Pestana M. Studies on the role of sodium on the synthesis of dopamine in the rat kidney. J Pharmacol Exp Ther 1993; 264(1): 406-14.
16. Soares-da-Silva P, Fernandes MH. Sodium-dependence and ouabain-sensitivity of the synthesis of dopamine in renal tissues of the rat. Br J Pharmacol 1992; 105(4): 811-6.
17. Pinho MJ, Serrao MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Renal Physiol 2007; 292(5): F1452-63.
18. Gomes P, Serrao MP, Viera-Coelho MA, Soares-da-Silva P. Opossum kidney cells take up L-DOPA through an organic cation potential-dependent and proton-independent transporter. Cell Biol Int 1997; 21(4): 249-55.
19. Vieira-Coelho MA, Hussain T, Kansra V et al. Aging, high salt intake, and renal dopaminergic activity in Fischer 344 rats. Hypertension 1999; 34(4 Pt 1): 666-72.
20. Pinho MJ, Serrao MP, Jose PA, Soares-da-Silva P. Organ specific underexpression renal of Na+-dependent B0AT1 in the SHR correlates positively with overexpression of NHE3 and salt intake. Mol Cell Biochem 2007; 306(1-2): 9-18.
21. Pinto V, Amaral J, Silva E et al. Age-related changes in the renal dopaminergic system and expression of renal amino acid transporters in WKY and SHR rats. Mech Ageing Dev 2011; 132(6-7): 298-304.
22. Jiang X, Zhang Y, Yang Y et al. Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of l-DOPA. Am J Physiol Endocrinol Metab 2017; 312(1): E1-E10.
23. Moura E, Silva E, Serrao MP, Afonso J, Kozmus CE, Vieira-Coelho MA. alpha2C-Adrenoceptors modulate L-DOPA uptake in opossum kidney cells and in the mouse kidney. Am J Physiol Renal Physiol 2012; 303(7): F928-38.
24. Kubovcakova L, Krizanova O, Kvetnansky R. Identification of the aromatic L-amino acid decarboxylase gene expression in various mice tissues and its modulation by immobilization stress in stellate ganglia. Neuroscience 2004; 126(2): 375-80.
25. Mappouras DG, Stiakakis J, Fragoulis EG. Purification and characterization of L-dopa decarboxylase from human kidney. Mol Cell Biochem 1990; 94(2): 147-56.
26. Hayashi M, Yamaji Y, Kitajima W, Saruta T. Aromatic L-amino acid decarboxylase activity along the rat nephron. Am J Physiol 1990; 258(1 Pt 2): F28-33.
27. Hayashi M, Yamaji Y, Kitajima W, Saruta T. Effects of high salt intake on dopamine production in rat kidney. Am J Physiol 1991; 260(5 Pt 1): E675-9.
28. Seri I, Kone BC, Gullans SR, Aperia A, Brenner BM, Ballermann BJ. Influence of Na+ intake on dopamine-induced inhibition of renal cortical Na(+)-K(+)-ATPase. Am J Physiol 1990; 258(1 Pt 2): F52-60.
29. Guimaraes JT, Vieira-Coelho MA, Serrao MP, Soares-da-Silva P. Opossum kidney (OK) cells in culture synthesize and degrade the natriuretic hormone dopamine: a comparison with rat renal tubular cells. Int J Biochem Cell Biol 1997; 29(4): 681-8.
30. Schoors DF, Velkeniers B, Dupont AG. A case of hyperdopaminuria due to increased renal dopamine production. Nephron 1990; 56(3): 329-31.
31. Fronzaroli C, La Villa G, Strazzulla G, Mannelli M, Franchi F. Renal effects of atrial natriuretic peptide during dopa-decarboxylase inhibition in patients with essential hypertension. Eur J Clin Pharmacol 1993; 44(5): 423-7.
32. Ball SG, Lee MR. The effect of carbidopa administration on urinary sodium excretion in man. Is dopamine an intrarenal natriuretic hormone? Br J Clin Pharmacol 1977; 4(2): 115-9.
33. Kopin IJ. Monoamine oxidase and catecholamine metabolism. J Neural Transm Suppl 1994; 4157-67.
34. Odlind C, Goransson V, Reenila I, Hansell P. Regulation of dopamine-induced natriuresisby the dopamine-metabolizing enzyme catechol-O-methyltransferase. Exp Nephrol 1999; 7(4): 314-22.
35. Zhang MZ, Yao B, McKanna JA, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol 2005; 288(4): F840-5.
36. Eklof AC, Holtback U, Sundelof M, Chen S, Aperia A. Inhibition of COMT induces dopamine-dependent natriuresis and inhibition of proximal tubular Na+,K+-ATPase. Kidney Int 1997; 52(3): 742-7.
37. Wang Y, Berndt TJ, Gross JM, Peterson MA, So MJ, Knox FG. Effect of inhibition of MAO and COMT on intrarenal dopamine and serotonin and on renal function. Am J Physiol Regul Integr Comp Physiol 2001; 280(1): R248-54.
38. Ball SG, Lee MR, Oates NS. The effect of inorganic salts on renal tissue dopamine levels in the rat [proceedings]. Br J Pharmacol 1978; 63(2): 343P.
39. Berndt TJ, MacDonald A, Walikonis R et al. Excretion of catecholamines and metabolites in response to increased dietary phosphate intake. J Lab Clin Med 1993; 122(1): 80-4.
40. Ibarra FR, Aguirre J, Nowicki S, Barontini M, Arrizurieta EE, Armando I. Demethylation of 3-O-methyldopa in the kidney: a possible source for dopamine in urine. Am J Physiol 1996; 270(5 Pt 2): F862-8.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/