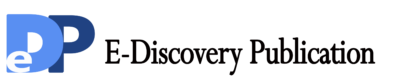J Med Discov (2018); 4(1):jmd18051; DOI:10.24262/jmd.4.1.18051; Received September 17th, 2019, Revised January 23th, 2019, Accepted January 28th, 2019, Published February 06th, 2019.
Research on the Effect of REM Sleep Deprivation and Revival on Rat
Lidao Bao 1,#, Lengge Si2,#, Sha Li1, Agula Bo2,*
1Department of Pharmacy, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, 010059, P. R. China
2College of Traditional Mongolia Medicine, Inner Mongolia Medical University, Hohhot, 010110, P. R. China
*Correspondence: Agula Bo, College of Traditional Mongolia Medicine, Inner Mongolia Medical University, Hohhot, 010110, P. R. China. Tel: 86-4713451717. Fax: 86-4713451717. Email: 349881704@qq.com.
#The first two authors contributed equally to this paper.
Abstract
Backgroun:Insomnia is a disease that is difficult to cure in modern medicine. Some supplemental and alternative medicines are effective. Mongolian medicine warm needle can treat insomnia in the past practice.
Objective:To study the changes in the expression of nNOS positive cells and the changes in the expression of nNOS mRNA during REM sleep deprivation at different points in time during sleep revival after deprivation.
Methods:The rats were divided into blank control group (CC), environmental control group (TC), Xingnaojing intervention group (XNJ) and the non-drug intervention group. The immunohistochemical method was used to test the expression level of the nNOS positive cells. The semiquantitative RT-PCR was used to determine the nNOS mRNA level.
Results: Compared with the control group, the memory ability of the rats in the sleep deprivation groups difference was significant (P<0.01); the expression of the nNOS positive cells increased and exacerbated with the pronged deprivation duration. Compared with the sleep deprivation group, the learning and memory abilities of the rats improved slightly at 6h, 12h, 24h, and 48h after sleep revival at 7 days after sleep deprivation.
Conclusion:Sleep deprivation causes increased expression of nNOS positive cells, produces a great deal of NO, inhibits the formation of long-term potentiation of the hippocampus, and deteriorates the learning and memory abilities. The nNOSmRNA expression level increases significantly with the duration of sleep deprivation. Xingnaojing plays a protective role in memory impairment arising from sleep deprivation.
Keywords: Sleep deprivation; Hippocampus; cortex; nNOS mRNA.
Introduction
Sleep deprivation refers to decreased sleep hours or declined sleep quality due to certain reasons. Sleep deprivation significantly affects human body. Sleep deprivation can cause adverse reactions such as fatigue, declined work capacity, deteriorated immunologic function, reduced memory ability, decreased work efficiency etc. [l,2]. Long-term sleep deprivation may lead to death. In recent years, the relation between sleep deprivation and cognitive disorder has been arousing more and more attention with rapid development such disciplines as chemical neuroanatomy, molecular pathology, cognitive neuroscience etc. [3]. Research shows that REM (rapid eye movement) sleep is significant to development and maturation of nervous systems of animals and closely associated with thermoregulation, autonomic nervous functions etc. [4,5]The cerebral oxygen consumption during the REM sleep period is significantly higher than that during strong physical and mental activity states and the cerebral protein synthesis would also increase significantly. Thus, it plays a very important role in advanced cerebral functions such as learning, memory, emotion etc. It is a major form of human body refreshment. In recent years, [6] research has shown that learning and memory are one of advanced cerebral functions, which includes a complex physiological and biochemical process. Their normal realization relies on integrity and coordination of relevant structures and functions. Exploration of the principles and regularities of learning and memory helps explain the mechanism of sleep disorders manifested by learning and memory disorders and find an effective approach for prevention and control of such disorders [7]. In recent years, many scholars have studied the mechanism of the effect of sleep deprivation on learning and memory but no definite conclusions have been obtained yet [8].
NO (Nitric-oxide) is a recently-identified special neurotransmitter [9]. It is a critical signal during the learning and memory process and can also cause severe nerve injury. As a new signal substance of the nervous system [10], NO participates in the long-term potentiation (LTP) mechanism related to synaptic plasticity and learning and memory with its unique retrograde messenger function. To further study the effect of the sleep deprivation on the learning and memory abilities, we observed the changes in the nitric oxide synthase (NOS) that was closely related to learning and memory at different points in time during sleep deprivation and at different points in time after sleep revival and observed the changes in the learning and memory abilities of the animals at different points in time after sleep revival by the water maze test and drug intervention. Then, we further study the changes in the expression of nNOS positive cells and the changes in the expression of nNOS mRNA during REM sleep deprivation at different points in time during sleep revival after deprivation and discuss the action mechanism of NO related to learning and memory disorders arising from sleep deprivation. Finally, we observed the effect of the sleep deprivation on learning and memory abilities and expression of nNOS positive cells by intervention with the drug of Xingnaojing, which provides a theoretical basis for further research on prevention and treatment of sleep deprivation.
Materials and Methods
Experimental animals and groups
100 male Sprague-Dawley rats aged weighing 250-280g were purchased from Nanjing Better Biotechnology Co., Ltd. The rats were randomly divided into the sleep deprivation (SD group, n=80) and the control group (n=20). The control group was subdivided into the blank control group (CC) and environmental control group (TC). The sleep deprivation group was subdivided into the Xingnaojing intervention group (XNJ) and the non-drug intervention group. Each group included 8 points in time, 1 day, 3 days, 5 days and 7 days after sleep deprivation and 6 hours, 12 hours, 24 hours, and 48 hours after sleep deprivation. There were 5 rats for each point in time. The rats were weighed and number individually.
Main reagents and instruments
Rabbit Anti-NOSl, ready-to-use SABC immunohistochemical staining kit, and DAB colour reagent (Wuhan Boster Biological Technology., LTD.), tissue/cell total RNA extraction kit ( Shanghai Huashun Bioengineering Co., Ltd.), MMuLV RT/PcR Kit, primers, tris, Agarose, (Shanghai Shenneng Bocai Biotechnology Co., Ltd.), internal reference B-actin (Shanghai Sangon Biotech Co., Ltd.), EDTA, glacial acetic acid (Shanghai Biochemical Reagent Co., Ltd.), PBS, TBS, etc. (prepared in the laboratory), Du 640 nucleic acid and protein analyzer (BECKMkN Company, U.S.), PCR amplifier (Techne Company, UK), electrothermal constant temperature water bath (Shanghai No.5 Medical Apparatus and Instrumentation Plant), electrophoresis apparatus (Beijing Liuyi Instrument Plant), electrophoresis analyzer, high-speed refrigerated centrifuge, MG-II maze (Shanghai Jiliang Software Technology Co.,Ltd.), CH optical microscope, BX-50 microscope, OLYMPUS BXS0 biological microscope (OLYMPUS Company, Japan), zD-Ⅲb fast biomedical experimental apparatus (Shanghai Kangcheng Biotechnology Co., Ltd.), HPIAS-1000 image measurement and analysis system (U.S. MediaCybermetic Company).
Establishment of REM sleep deprivation models
The modified multiple platform REM sleep deprivation method was used to establish a REM sleep deprivation model [11]. The rats woke after nutation and touching the water thus always preventing the animal from entering the REM sleep period and leading to REM sleep deprivation. The muscle tone of the rats decreased when they enter the REM sleep period. The rats stayed on the platform for 1 week (1h each day) before the experiment. The TC group used the rat boxes with the size same as that used by the SD group but a closely-woven wire net was placed at the bottom instead of a platform. The rats were placed on the net. Water was placed 1cm under the net to form an environment similar to that in the SD group. Other conditions were similar to those in the SD group. In the CC group, there were rats that were raised for 2 weeks and received no treatment. The raising conditions were the same as that in the SD group and TC group.
Test of Cognitive Functions
Setup parameters of the MG-Ⅱ “Y” maze (Shanghai Jiliang Technology Co., Ltd.) [12]. Electrical Stimulation Parameters: Voltage 50-70v, time delay 2-5 s. It was a right response that the rats ran to the safe area within 10s after their feet were electrified. The rats were trained for 3-4 consecutive days, 20 times each day. It was defined as a “right response” that the rats ran directly from the staring area to the safe area after electric shock. The learning and memory performance of the rats was expressed with the times of electric shock required for 9 consecutive right ones of the 10 responses. The times of electric shock required for 9/10 right responses indicated the quality of the cognitive functions of the rats.
Immunohistochemical staining
The animals in the immune group were sacrificed at corresponding points in time [13]. The brain was placed in 30% saccharose. The sections were regularly dewaxed and washed 3 times with distilled water. The antigen was subject to thermal remediation. The sections were blocked at room temperature for 20 minutes after addition of normal goat serum blocking buffer (Germany Calbiochcm Company). The sections were cultured at 37℃ for 1 hour after addition of 1:300 diluted rabbit anti neuronal nitric oxide synthase (Germany Calbiochcm Company). The sections were cultured at 37℃ for 20 minutes after addition of biotinylated goat anti-mouse IgG (U.S. Jackson Company). The sections were cultured at 37℃ for 20 minutes after addition of SABC (prepared in the laboratory). 1 ml of distilled water and 1 droplet of color developing agents of A, B, and C were well mixed and added to the sections. The sections were dehydrated, cleared, mounted, and observed under a microscope. The positive products from immunoreaction were counted using the standard grid under a 40X light microscope with 3 adjacent fields.
Extraction of total RNA in rat brain
The brain was taken immediately after the rat was decollated [14]. It was placed in the Eppendorf tube treated with 0.1% DEPC and cryopreserved at – 70℃ after the bilateral cortices and hippocampi were separated. Extraction of total RNA used the small-amount tissue/cell total RNA extraction reagent kit produced by Shanghai Huashun Bioengineering Co., Ltd. Specific plans were implemented as per the instructions for the kit.
Determining the nNOS mRNA level using semiquantitative RT-PCR
The rat nNOS genetic cDNA sequence was found in the gene library. The ligo 5.0 software program was used to design sense and antisense primers:
Upstream primer RF: 5’GGGGCTCAAATGGTATGG3’;
Downstream primer PR: 5’GATGAAGGACTCCGTGGC3’.
Upstream primer RF: 5’TGTATGCCTCTGGTCGTACCAC3’;
Downstream primer :
PR:5’ACAGAGTACTTGCGCTCAGGAG’.
Total RNA of a sample was taken and placed in 6 tubes (2μl for each tube). It was subject to RT-PCR amplification at the same time. One tube was taken every other 3 cycles from the 20th cycle until the 35th cycle. The product was stained with 0.5% μg/100ml ethidium bromide for 7min after electrophoresis with 1.5% agarose. The electrophoresis result subject to an electrophoretic band analysis with the GIS digital gel image processing system. A diagram was plotted with the ratio of NOS to β-actin absorption peak area v.s. number of amplification cycles. The most appropriate number of cycles was selected. 0.25, 0.5, 0.75, 1.0, 1.25, and 1.50μg of the total RNA of one sample served as templates for PCR amplification. A diagram was plotted with the ratio of NOS to β-actin absorption peak area v.s. number of amplification cycles. The most appropriate number of templates was selected.
Statistical Method
The enumeration data were expressed with. The difference significance was subject to a t test analysis. The relative expression level of nNOS mRNA was calculated with nNOS/β-actin. The experimental data were expressed with. The data were subject to a variance analysis and q test with the SPSS19.0 statistical software. P<0.05 indicated that the difference was significant.
Results
Determination of learning and memory
No learning and memory disorders occurred to the normal control group and the environmental control group from beginning to end. Compared with the control group, the times of electric shock increased significantly (P<0.01) with the increase of the deprivation duration and learning and memory disorders occurred in the sleep deprivation group. The learning and memory abilities improved with the extension of the revival time after sleep deprivation. Compared with the non-intervention sleep deprivation group, the electric shock times were fewer and the learning and memory abilities were better in the Xingnaojing group. However, the electric shock times increased with the deprivation duration; the learning and memory abilities declined; the learning and memory abilities improved with the increasing revival time after sleep deprivation. See Figure 1 for the determination result.
Fig. 1 Learning and memory performance of rats in the Y-Type maze experiment. *Compared with the control group, P<0.05. **Compared with the control group, P<0.01.
Immunohistochemical staining
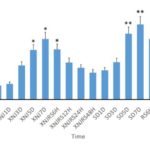
The nNOS immune positive cells exhibited a brownish yellow color after the sections of various groups were subject to immunohistochemical staining (Figure 2). Compared with the control group, the nNOS immune positive cells in the hippocampi and cortical areas of the rats in the REM sleep deprivation group The statistical result has indicated that the NA level in the REM sleep deprivation group is significantly higher than that in the environmental control group and the blank control group (P<0.01). The NA level of the nNOS positive cells in the Xingnaojing group was lower than that in the REM sleep deprivation group and also significantly higher than that in the environmental control group and the blank control group (P<0.01) (Figure 3).
Changes in the expression of nNOS mRNA in the hippocampus and cortex after REM sleep deprivation and revival
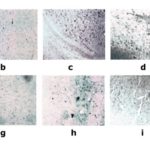
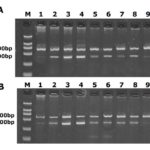
After agarose gel electrophoresis, it was observed that the two bands of nNOS and β-actin were 307 bp and 592 bp respectively (Figure 4). There was no significant difference in the expression of nNOS mRNA in the hippocampus and cortex in the CC group and TC group. The expression of nNOS mRNA increased at 1 day and 3 days after REM sleep deprivation. The expression of nNOS mRNA increased significantly at 5 days after REM sleep deprivation. The expression of nNOS mRNA reached the peak value at 7 days after REM sleep deprivation. The expression of nNOS mRNA was still higher than that in the TC group at 6 hours, 12 hours, and 24 hours after sleep revival (Table 1).
Table 1. Changes in the Expression of nNOS mRNA in the Hippocampus and Cortex after REM Sleep Deprivation and Revival
| Group | Hippocampus | Cortex |
| CC | 0.484±0.025 | 0.435±0.022 |
| TC | 0.526±0.024 | 0.492±0.021 |
| SD1d | 0.611±.0.21 | 0.604±0.003 |
| SD3d | 0.604±0.024 | 0.653±0.011 |
| SD5d | 0.650±0.011* | 0.654±0.022* |
| SD7d | 0.672±0.023* | 0.674±0.014* |
| RS6h | 0.563±0.012 | 0.662±0.012 |
| RS12h | 0.531±0.007 | 0.609±0.014 |
| RS24h | 0.595±0.019 | 0.589±0.023 |
| RS48h | 0.482±0.012 | 0.573±0.020 |
Legend: Compared with the environmental control group, * P<0.05.
Fig. 2 Pictures of Sections in Various Groups after Immunohistochemical Staining with nNOS. a. presents the expression of nNOS positive cells in the hippocampus at 5 days after sleep deprivation; b. presents the expression of nNOS positive cells in the hippocampus at 7 days after sleep deprivation; c. presents the expression of nNOS positive cells in the cortex at 5 days after sleep deprivation; d. presents the expression of nNOS positive cells in the cortex at 7 days after sleep deprivation; e. presents the expression of nNOS positive cells in the hippocampus at 5 days after sleep deprivation in the Xingnaojing group; f. presents the expression of nNOS positive cells in the hippocampus at 7 days after sleep deprivation in the Xingnaojing group; g. presents the expression of nNOS positive cells in the cortex at 5 days after sleep deprivation in the Xingnaojing group; h. presents the expression of nNOS positive cells in the cortex at 7 days after sleep deprivation in the Xingnaojing group; i. presents the expression of nNOS positive cells in the hippocampus at 5 days after sleep deprivation in the blank control group; j. presents the expression of nNOS positive cells in the hippocampus at 7 days after sleep deprivation in the blank control group.
Discussion
Sleep is a normal physiological need of organisms [15,16]. Sleep deprivation will produce corresponding physical and psychological effects on organisms and lead to behavior disorder and incidence of diseases [17]. REM sleep has greater effect on learning and memory during the sleep stage. The amount of REM sleep increased significantly after learning complex knowledge or completing many tasks [18,19]. The amount of REM sleep increased slightly after completing simple tasks or learning simple knowledge. Exploration of the principles and regularities of learning and memory helps explain the mechanism of sleep disorders manifested by learning and memory disorders and find an effective approach for prevention and control of such disorders [20]. Nitric oxide synthase (NOS) is the only enzyme of endogenous from catalyzing [21,22]. It largely determines the biological effect of NO as a result of such characteristics of NO as non-vesicle release, high dispersion, extremely short half life. As a new signal substance of the nervous system, NO participates in the long-term potentiation mechanism related to synaptic plasticity and learning and memory with its unique retrograde messenger function.
NO is a recently-identified special neurotransmitter [23,24]. It is a critical signal during the learning and memory process. It can also cause severe nerve injury. NO is related to the formation of long-term potentiation of the hippocampus. Nitric oxide is generated from L-arginine and molecular oxygen under the catalysis of 3 different nitric oxide synthases. Based on the cellular or histological origins, the enzymes can be divided into 3 types: neuronal NOS(nNOS, NOS-1), immune NOS (iNOS, NOS-2), and endothelial cellular NOS(ecNOS, NOS-3). Based on the functions, nNOS and ecNOS constitutive ones (cNOS) and iNOS is an inducible one (iNOS). It is generally accepted that [25-28] the NO produced by ecNOS has neuroprotective effect while the NO produced by nNOS and iNOS has neurotoxic effect.
It has been discovered in the experiment that compared with the control group, the learning performance of all sleep-deprived rats decline and the learning ability of the rats declines significantly with the increasing sleep deprivation duration. Extension of sleep deprivation duration leads to an increase in the number of the nNOS positive cells. Catalysis generates high-concentration NO. The high-concentration NO has neurotoxic effect. It can induce oxidative stress, inhibit the functions of mitochondria, interfere with energy metabolism, damage DNA or cause DNA mutation, increase the Ca2+ in cytosol, damage cells, and induce cell apoptosis. Extremely-high NOS also has neurotoxic effect [29]. The number of the NOS positive cells decreases and the learning and memory abilities are excellent in the Xingnaojing group, which may be related to the special pharmacological action of Xingnaojing compared with that in the non-intervention group.
Fig. 3 A.Comparison of NA Values of nNOS Immune Positive Cells in the Hippocampus;B.Comparison of NA Values of nNOS Immune Positive Cells in the Cortical Region.Comparison of NA in nNOS Positive Cells in the Hippocampus and Cortex Regions Compared with the control group, P<0.05; ** compared with the control group, P<0.01.
The experiment has indicated that sleep deprivation after injection with Xingnaojing can improve the learning and memory abilities of the rats, decrease the electric shock times, and decline the number of the nNOS positive cells compared with that in the non-intervention sleep deprivation group. The early application of Xingnaojing can improve the damage to learning and memory after sleep deprivation. Its mechanism may be related to the expression of certain cell factors and may also be relevant to antagonism and NO expression. Meanwhile, it can improve intracellular metabolic disorders thus improving learning and memory disorders, which indicates that Xingnaojing has protective effect on memory impairment arising from sleep deprivation.
Fig. 4 Electrophoresis Result a presents the RT-PCR products from agarose gel electrophoresis of the hippocampus. The two bands are nNOS and β-actin respectively. 1-9 are 1d, 3d, 5d and 7d after sleep deprivation, 6h, 12h, 24h, 48h after sleep revival, CC group and TC group; b presents the RT-PCR products from agarose gel electrophoresis of the cortex; the two bands are nNOS and β-actin; 1-9 are 1d, 3d, 5d and 7d after sleep deprivation, 6h, 12h, 24h, 48h after sleep revival, CC group and TC group.
The experiment result has indicated that sleep deprivation can not only lead to the expression of the nNOSmRNA level and the expression level of nNOSmRNA can be increased significantly with the sleep deprivation duration. The expression level of nNOSmRNA declines significantly with the increasing time after sleep revival. It demonstrates that the nNOS closely related to learning and memory is affected by sleep deprivation. It coincides with the fact that corresponding changes occur to the memory function based on the Y maze test. The experiment has indicated that the prolonged sleep deprivation duration causes increased expression of nNOSmRNA and produces a great deal of nNOS. Catalysis produces high-concentration NO. The high-concentration NO has toxic effect on the nerves. It induces oxidative stress, inhibits mitochondrial functions, interferes with energy metabolism, damages DNA or leads to DNA mutation, raises the level of Ca2+ in cytosol, and interferes with the LTP process. It can also a decline in the learning and memory abilities. The nNOSmRNA expression decreases at different points in time during sleep revival after sleep deprivation. The sleep revival can mobilize various types of potential of the mechanisms, balance the concentrations of various neurotransmitters, and remove excessive NO. The NO at a physiological concentration plays a role. It activates the ornithine cyclase through cytomembranein, raises the cGMP level, and promotes release of the neurotransmitters (such as glutamic acid etc.). The glutamic acid acts on the postsynaptic NMDA and non-NMDA receptor. It also induces the LTP process. The experiment involves research on the changes in the expression of the NOSgene level with the RT-PCR technique, which provides a theoretical basis for further exploration of the mechanism for the effect of sleep on the learning and memory abilities.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Acknowledgments
This research is funded by the following projects: National Natural Science Foundation of China-(No. 81560801);Science and Technology Innovation Fund of Provincial Department of Finance, Inner Mongolia Autonomous Region; Collaborative Innovation Project of Mogolian Medicine, Inner Mongolia Autonomous Region; Technology Reserve Project of Provincial Department of Science and Technology, Inner Mongolia Autonomous Region.Inner Mongolia Autonomous Region Mongolian Medicine Cooperative Innovation Project.Inner Mongolia Autonomous Region “Prairie excellence”Project.
References
1. McDevitt EA, Rowe KM, Brady M, Duggan KA, Mednick SC. The benefit of offline sleep and wake for novel object recognition. xperimental brain research. 2014;232(5):1487-96.
2. Spoormaker VI, Gvozdanovic GA, Samann PG, Czisch M. Ventromedial prefrontal cortex activity and rapid eye movement sleep are associated with subsequent fear expression in human subjects. Experimental brain research. 2014;232(5):1547-54.
3. McCarley RW. Neurobiology of REM and NREM sleep. Sleep medicine. 2007;8(4):302-30.
4. Zeeuw J, Wisniewski S, Papakonstantinou A, Bes F, Wahnschaffe A, Zaleska M, et al. The alerting effect of the wake maintenance zone during 40 hours of sleep deprivation. Scientific reports. 2018;8(1):11012.
5. Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787-98.
6. Kenyon KA, Bushong EA, Mauer AS, Strehler EE, Weinberg RJ, Burette AC. Cellular and subcellular localization of the neuron-specific plasma membrane calcium ATPase PMCA1a in the rat brain. The Journal of comparative neurology. 2010;518(16):3169-83.
7. Rudolph U, Mohler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annual review of pharmacology and toxicology. 2014;54:483-507.
8. Iqbal F, Ellwood R, Mortensen M, Smart TG, Baker JR. Synthesis and evaluation of highly potent GABA(A) receptor antagonists based on gabazine (SR-95531). Bioorganic & medicinal chemistry letters. 2011;21(14):4252-4.
9. Schwab FJ, Blondel B, Bess S, Hostin R, Shaffrey CI, Smith JS, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine. 2013;38(13):E803-12.
10. Di Giacopo R, Fasano A, Quaranta D, Della Marca G, Bove F, Bentivoglio AR. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2012;27(4):559-61.
11. Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297-308.
12. Wei H, Huang JL, Hao B, Wang YC, Nian G, Ma AN, et al. Intrathecal administration of antioxidants attenuates mechanical pain hypersensitivity induced by REM sleep deprivation in the rat. Scandinavian journal of pain. 2011;2(2):64-9.
13. Liu YP, Tung CS, Lin YL, Chuang CH. Wake-promoting agent modafinil worsened attentional performance following REM sleep deprivation in a young-adult rat model of 5-choice serial reaction time task. Psychopharmacology. 2011;213(1):155-66.
14. Ting CH, Huang HN, Huang TC, Wu CJ, Chen JY. The mechanisms by which pardaxin, a natural cationic antimicrobial peptide, targets the endoplasmic reticulum and induces c-FOS. Biomaterials. 2014;35(11):3627-40.
15. Shehab S, D’Souza C, Ljubisavljevic M, Redgrave P. High-frequency electrical stimulation of the subthalamic nucleus excites target structures in a model using c-fos immunohistochemistry. Neuroscience. 2014;270:212-25.
16. Deng Y, Wang W, Yu P, Xi Z, Xu L, Li X, et al. Comparison of taurine, GABA, Glu, and Asp as scavengers of malondialdehyde in vitro and in vivo. Nanoscale research letters. 2013;8(1):190.
17. Fievisohn EM, Sajja VS, Vandevord PJ, Hardy WN. Evaluation of impact-induced traumatic brain injury in the Gottingen Minipig using two input modes. Traffic injury prevention. 2014;15 Suppl 1:S81-7.
18. Ganji SK, An Z, Banerjee A, Madan A, Hulsey KM, Choi C. Measurement of regional variation of GABA in the human brain by optimized point-resolved spectroscopy at 7 T in vivo. NMR in biomedicine. 2014;27(10):1167-75.
19. Black SW, Morairty SR, Fisher SP, Chen TM, Warrier DR, Kilduff TS. Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. Sleep. 2013;36(3):325-36.
20. Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, et al. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(12):5275-84.
21. Kwon Jeong J, Dae Kim J, Diano S. Ghrelin regulates hypothalamic prolyl carboxypeptidase expression in mice. Molecular metabolism. 2013;2(1):23-30.
22. Liu C, Liu X, Liu S. Bifurcation analysis of a Morris-Lecar neuron model. Biological cybernetics. 2014;108(1):75-84.
23. Sivakumar SS, Namath AG, Galan RF. Spherical Harmonics Reveal Standing EEG Waves and Long-Range Neural Synchronization during Non-REM Sleep. Frontiers in computational neuroscience. 2016;10:59.
24. Drakatos P, Patel K, Thakrar C, Williams AJ, Kent BD, Leschziner GD. Sleep-stage sequencing of sleep-onset REM periods in MSLT predicts treatment response in patients with narcolepsy. Journal of sleep research. 2016;25(2):203-10.
25. Descamps A, Rousset C, Dugua H, Debilly G, Delagrange P, Cespuglio R. Agomelatine restores a physiological response to stress in the aged rat. Neuroscience letters. 2014;566:257-62.
26. Duce B, Kulkas A, Langton C, Toyras J, Hukins C. The prevalence of REM-related obstructive sleep apnoea is reduced by the AASM 2012 hypopnoea criteria. Sleep & breathing = Schlaf & Atmung. 2018;22(1):57-64.
27. Al Oweidat K, AlRyalat SA, Al-Essa M, Obeidat N. Comparing REM- and NREM-Related Obstructive Sleep Apnea in Jordan: A Cross-Sectional Study. Canadian respiratory journal. 2018;2018:9270329.
28. Nguyen TQ, Liang CL, Marks GA. GABA(A) receptors implicated in REM sleep control express a benzodiazepine binding site. Brain research. 2013;1527:131-40.
29. Wei H, Hao B, Huang JL, Ma AN, Li XY, Wang YX, et al. Intrathecal administration of a gap junction decoupler, an inhibitor of Na(+)-K(+)-2Cl(-) cotransporter 1, or a GABA(A) receptor agonist attenuates mechanical pain hypersensitivity induced by REM sleep deprivation in the rat. Pharmacology, biochemistry, and behavior. 2010;97(2):377-83.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/