J Med Discov (2019); 4(1):jmd18045; DOI:10.24262/jmd.4.1.18045; Received November 19th, 2018, Revised December 16th, 2018, Accepted December 21st, 2018, Published January 10th, 2019.
Alteration in the levels of some markers of Oxidative Stress and liver function induced by Tramadol Administration in Male Rabbits: the effect of its withdrawal
Mathias Abiodun Emokpae1, Oluwabusayo Bunmi Ogunniyi2, Gideon Olumide Dada2, Vincent Ifeoluwa Awopetu2
1Department of Medical Laboratory Science, School of Basic Medical Sciences, College of Medical Sciences, University iof Benin, Benin City, Nigeria.
2Department of Medical Laboratory Science, College of Natural and Applied Sciences, Achievers University, Owo, Ondo State, Nigeria
* Correspondence: M.A Emokpae, Department of Medical Laboratory Science, School of Basic Medical Sciences, University of Benin, Benin City.
Email:mathias.emokpae@uniben.edu
Abstract
Tramadol is an opiod pain medication used in the management of moderate to severe pain. Chronic administration of tramadol in pain management and its use as an acceptable alternative in individuals with drug seeking behaviours is controversial. Thus the aim of this study was to investigate the changes in some markers of oxidative stress and renal function in rabbits on acute and chronic administration of Tramadol. A total number of 24 male rabbits were used for this study; divided into 8 groups of 3 rabbits each based on similarities in weight and administered with varying doses of tramadol. Groups A, B and C were given 10, 15 and 20mg/kg body weight of tramadol intramuscularly for 15days while group D and E were given 10 and 20mg/kg body weight intramuscularly for 30days. Group F and G were given 10 and 20mg/kg body weight for 15 days followed by tramadol withdrawn and observed for 15days while group H was given only water and feeds(controls). Blood samples were collected from vein lining the ear of the rabbits at the end of drug administration. Plasma level of urea, creatinine, malondialdehyde (MDA), uric Acid, aspartate amino transferase (AST), alanine amino transferase (ALT) and alkaline phosphatase (ALP) were determined using spectrophotometric techniques. The mean levels of urea, creatinine, MDA, uric acid, AST, ALT and ALP were significantly higher (p<0.001) in tramadol treated rabbits than control, with levels increasing with increased concentrations of administered tramadol. Even though the mean levels of measured parameters decreased upon withdrawal of treatment, the levels were still significantly higher than controls. In conclusion, acute and chronic administration of tramadol might induce oxidative stress, hepatic and renal impairments.
Keywords: Tramadol, malondialdehyde, oxidative stress and renal function.
Introduction
Tramadol is a popular pain killer and is commonly abused leading to mortality and morbidity of subjects, It acts by inhibiting neurotransmitters such as dopamine, noradrenaline and acetylcholine. Tramadol exerts its pain killing effect by two complimentary and synergistic mechanisms: by activating the µ-opioid receptor [1] and by inhibition of the neuronal uptake of noradrenaline and serotonin [2]. Tramadol is considered a safe drug devoid of many serious adverse effects of traditional opioids. However, abuse and dependence on tramadol as well as toxicity and tramadol related deaths have been increasingly reported [3]. Repeated tramadol administration in such patients might lead to the accumulation of toxic metabolites in the body, increase the risk for pharmacokinetic interactions, and/or decrease the clearance of tramadol, thus increasing its potential for toxicity [4]. Nowadays addiction is an ever-increasing problem in the world and despite efforts to prevent and control it, it continues to be a tremendous public health issue. Analgesics are among the most popular drugs which are being abused [5].
Oxidative stress occurs when there is an imbalance between pro-oxidants (free radicals) generation and the antioxidants system resulting in the damage to lipids and biomolecules [6]. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by reactive oxygen species (ROS) generated such as superoxide radical, hydroxyl radical and hydrogen peroxide [7]. The effects of oxidative stress depend upon the size of these changes, with a cell being able to overcome small perturbations and regain its original state. However, more severe oxidative stress can cause cell death, and even moderate oxidation can trigger apoptosis, while more intense stresses may cause necrosis [8]. Production of reactive oxygen species is a particularly destructive aspect of oxidative stress.
Lipid peroxidation is a chain reaction occurring during oxidative stress leading to the formation of various active compounds including propanedial and 4-hyrdoxynonenal (HNE) resulting in the cellular damage. Lipid peroxidation can be initiated by any chemical species that can extract a hydrogen atom from side chain of a polyunsaturated fatty acid (PUFA) which is generally present in the cell membranes. Arachidonic acid is a polyunsaturated omega-6 fatty acid present in the cell membranes, brain, muscles and liver that contains many uninterrupted methylene double bonds that serve as a source of hydrogen atoms for the free radical reactions. Arachidonic acid induces the platelets to produce large amounts of malondialdehyde (MDA)[7].
The liver and kidney are responsible for the metabolism of tramadol. Therefore, may cause hepatotoxicity and nephrotoxicity during chronic administration [9,10]. Since the kidney and liver are the primary excretory and metabolizing organs for drugs, chemicals and biotransformed products, they may be vulnerable to toxicity and these calls for concern during chronic administration of drugs. Therefore, the need for constant clinical assessment during therapy with drugs to safeguard them from toxicity cannot be overemphasized [11].
However, recent abuse and dependence of tramadol as well as toxicity are increasingly reported [3]. Thousands of young men are developing dependence to a prescription painkiller used to alleviate the stress of living. They also use it to relieve psychosomatic symptoms such as headaches and abdominal pain, as well as depression and nervousness [12]. Students, laborers, and even professionals are addicted to tramadol in our setting. The awareness of the dangers inherent in the abuse of tramadol appears to be lacking. This study may help create the most needed evidence based information to bridge this gap. This study was therefore aimed to determine the changes in some markers of oxidative stress and renal function in rabbit on acute and chronic administration of Tramadol and after withdrawal.
Materials and Methods
This experimental and observational study involving 24 rabbits was conducted at the animal house of Achievers University, Owo, Nigeria.
Experimental Animals
Twenty-four male rabbits, Oryctolagus cuniculus was purchased in Owo through the Department of Biological Science, Achievers University, Owo – Nigeria and housed in ventilated cages at the animal house, Department of microbiology Achievers university Owo. The animals were allowed to acclimatize for one week during which they were fed with grower’s mash and water ad libitum. The 24 rabbits were divided into 6 groups of 3 rabbits each. The animals were grouped based on similarities in weight and were fed with the appropriate food and tap water provided for ad libitum for drinking which was renewed every day. Body weight of animal before and after the experiment was measured using weighing balance. The dosage range of tramadol used was as suggested by Analgesic Drugs for use in rabbits 2003-2018, but higher dosages 15-30mg/kg reflect over dose (www.medrabbit.com).
GROUP A: 3 rabbits were fed with normal meal and injected daily with tramadol (10mg/kg. body weight) for15 days
GROUP B: 3 rabbits were fed with normal meal and injected daily with tramadol (15mg/kg body weight) for 15 days
GROUP C: 3 rabbits were fed with normal meal and injected daily with tramadol (20mg/kg body weight) for 15 days.
GROUP D: 3 rabbits were fed with normal meal and injected daily with tramadol (10mg/kg body weight) for 30 days.
GROUP E: 3 rabbits were fed with normal meal and injected daily with tramadol (20mg/kg body weight) for 30 days.
GROUP F: 3 rabbits were fed with normal meal and injected daily with tramadol (10mg/kg body weight) for 15 days and allowed to recover for 15 days.
GROUP G: 3 rabbits were fed with normal meal and injected daily with tramadol (20mg/kg body weight) for 15 days and allowed to recover for 15 days.
CONTROL GROUP
Group H: 3 rabbits were fed with normal meal.
Blood sample collection
Five millilitres of blood sample was collected from the vein lining the ear of each rabbits into lithium heparinized bottles after completion of treatment. The whole blood sample in lithium heparinized bottle was centrifuged at 1000g for 10 minutes and plasma separated into plain container. The separated plasma was kept frozen at -20 degree Celsius until analyzed.
Sample Analyses
Plasma level of uric acid, urea, creatinine, malondialdehyde, aspartate amino transferase (AST), alanine amino transferase (ALT) and alkaline phosphatase (ALP) were determined by spectrophotometric method. Malondialdehyde was assayed using the thiobarbituric acid reactive substance (TBARS), uric acid is determined by using Uricase method while urea and creatinine were assayed using Urease Berthelot and Jaffes methods respectively. The serum AST, ALT and ALP were assayed using reagents by Randox Laboratories.
Malondialdehyde Principle: This method is based on the reaction between 2-thiobabituric acid (TBA) and malondialdehyde, an end product of lipid peroxidation. On heating in an acidic pH, the product is a pink colour complex which is absorb maximally at 523nm and which is extractable into organic solvents such as butanol. The results are expressed as a result of free malondialdehyde produced.
Uric acid Principle: Uric acid is oxidized by uricase to allantoin and hydrogen peroxide. The hydrogen peroxide reacts with polyhydrogenaated benzoic acid (PHBA) and 4-aminoantipyrine (4-AAP) in the presence peroxidase (Hydrogen peroxidoreductase) to yield a quinoneimine dye (chromogen). The intensity of the dye is measured directly and is directly proportional to the concentration of uric acid present in the sample.
Urea Principle: Urea is split into ammonia and carbon dioxide by the action of urease, an enzyme obtained from Jack Bean meal. The ammonia then reacts with alkaline hypochlorite and phenol in the presence of a catalyst-sodium nitroprusside. The resulting product is indophenol (a blue color) and the concentration of ammonia is directly proportional to the absorbance of indophenol.
Creatinine Principle: Creatinine in alkaline solution reacts with picric acid to form a colored complex. The amount of the complex formed is directly proportional to the creatinine concentration.
ALT Principle: ALT catalyzes the transfer of an amino group from L-aspartate to 2-oxoglutarate to form oxaloacetate and L-glutamate. Oxaloacetate spontaneously decarboxylates to form pyruvate under strongly acidic conditions.
AST Principle: AST catalyzes the transfer of an amino group from L-aspartate to 2-oxoglutarate to form oxaloacetate and L-glutamate.
ALP Principle: The substrate of the p-nitrophenyl phosphate is hydrolyzed by Alkaline Phosphatase from the sample in the presence of magnesium ions, to form p-nitrophenol is a yellow in colour. The intensity of the colour is proportional to the Alkaline Phosphatase activity in the sample.
Statistical Analysis
Statistical analysis was done using the Statistical Package for Social Scientists (SPSS, Chicago, IL, USA) version 20.0. Values were expressed as Mean± Standard deviation. Results from the specimen were compared using Analysis of variance (ANOVA). Level of significance was taken at 95% confidence interval and P value less 0.05 was considered significant.
Results
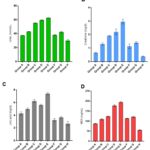
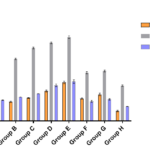
The results obtained are showed in tables 1-4 and figures 1and 2. The levels of urea, creatinine, uric acid, MDA, AST, ALT and ALP were significantly higher (p<0.001) in all experimental groups of tramadol treated rabbits than control group except AST in group A which was significantly higher (p<0.005) than controls. Upon withdrawal of tramadol treatment, the levels of all measured parameters were still significantly higher (p<0.001) than controls. The levels of the measured parameters in recovery group (Group F and G) failed to return to the levels observed in control rabbits. The mean levels of measured variables increased with increasing concentrations of administered tramadol. The mean levels of urea (r=0.472; p<0.02), uric acid (r=0.392; p<0.05), MDA (r= 0.72; p<0.05), AST (r=0.29;p<0.05), ALT (r=0.399;p<0.05) and ALP (r=0.391;p<0.05) correlated positively with concentration of administered tramadol. In the same vein, the mean levels of urea(r= 0.469; p<0.02), uric acid (0.412; p<0.05), MDA (r= 0.407; p<0.05), AST (r=0.410;p<0.05), ALT (r=0.58;p<0.06) and ALP (0.312;p<0.07) correlated with duration of tramadol treatment.
Table 1: Comparison of measured variables in animals treated with Tramadol and Controls
|
GROUPS OF ANIMALS |
UREA (mmol/L) |
Creatinine (mg/dl) |
Uric acid (mg/dl) |
MDA (mg/mL) |
|
A(10mg/kg body weight for 15days |
37.47±0.21 ** |
0.65±0.04** |
4.3±0.25** |
88.5±1.5** |
| B(15mg/kg body weight for 15days) | 42.97±0.12** | 1.3±0.08** | 5.0±0.16** | 110±3.8** |
| C(20mg/kg body weight for 15days) | 55.47±0.37** | 1.91±0.032** | 6.26±0.21** | 124±2.6** |
| D(10mg/kg body weight for 30days) | 59.7±0.25** | 2.2±0.21** | 5.63±0.12** | 178±4.2** |
| E(20mg/kg body weight for 30days | 62.73.±0.21** | 2.97±0.17** | 7.4±0.16** | 195±3.9** |
| F(10mg/kg body weight for 15days and withdrawn treatment for 15days | 37.93±0.78** | 1.13±0.17** | 3.27±0.21** | 114±1.6** |
| G(20mg/kg body weight for 15days and withdrawn treatment for 15days | 42.13±1.2** | 1.4±0.08** | 3.67±0.13** | 122±3.3** |
| H(Controls) | 30±1.63 | 0.39±0.029 | 2.7±0.34 | 55.8±2.1 |
** P<0.001 when compared with control. MDA=malondialdehyde
Table 2: Comparison of measured markers of liver function in animals treated with Tramadol and Controls
|
GROUPS OF ANIMALS |
AST (IU/L) |
ALT (IU/L) |
ALP (IU/L) |
|
A(10mg/kg body weight for 15days |
52.1±4.2* |
172±4.8** |
72.4±0.7** |
| B(15mg/kg body weight for 15days) | 66.45±2.35** | 216±2.5** | 83.1±0.29** |
| C(20mg/kg body weight for 15days) | 79.80±1.25** | 25 4±3.6** | 94.8±0.2** |
| D(10mg/kg body weight for 30days) | 104±3.6** | 272±2.9** | 124±4.8** |
| E(20mg/kg body weight for 30days | 134±4.2** | 292±4.6** | 137±6.3** |
| F(10mg/kg body weight for 15days and withdrawn treatment for 15days | 78±2.3** | 168±3.9** | 68±3.7** |
| G(20mg/kg body weight for 15days and withdrawn treatment for 15days | 92±4.7** | 174±3.1** | 75±2.8** |
| H(Controls) | 34.7±2.8 | 123±2.3 | 50.3±0.3 |
*(p<0.005); **(p<0.001); AST=Aspartate amino transferase; ALT=Alanine amino transferase; ALP=Alkaline phosphatase.
Table 3: Association of measured variables with concentrations of administered tramadol
| Measured variables | R-value | P-value |
| Urea | 0.472 | 0.02 |
| Creatinine | 0.312 | 0.15 |
| Uric acid | 0.392 | 0.05 |
| Malondialdehyde | 0.398 | 0.05 |
| Aspartate amino transferase | 0.429 | 0.05 |
| Alanine amino transferase | 0.399 | 0.05 |
| Alkaline phosphatase | 0.391 | 0.05 |
Table 4: Correlation of measured parameters with duration of tramadol treatment
| Measured parameters | R-value | P-value |
| Urea | 0.469 | 0.02 |
| Creatinine | 0.215 | 0.5 |
| Uric acid | 0.412 | 0.05 |
| Malondialdehyde | 0.407 | 0.05 |
| Aspartate amino transferase | 0.410 | 0.05 |
| Alanine amino transferase | 0.358 | 0.06 |
| Alkaline phosphatase | 0.312 | 0.07 |
Figure 1: Graphical representation of urea (A), creatinine (B), uric acid(C) and malondialdehyde (D) levels in experimental rabbits.
Figure 2: Histogram of the activities of liver enzymes in experimental rabbits
Discussion
The result obtained from this study showed that all measured parameters were significantly higher in rabbits administered with tramadol than controls and mean levels of parameters in rabbits upon withdrawal of treatment for 15days were still higher than controls. Tramadol hydrochloride is one of morphine derivatives; widely used opioid in recent years as an effective analgesic agent for the treatment of acute or chronic pain [13]. It is an analgesic of significant and has wide ranging application mostly in the treatment of moderate to severe pain including the treatment of fibromyalgia, cancer and musculoskeletal pain. Tramadol overdosing has been one of the most frequent causes of drug poisoning in the recent years, especially in young adults with a history of substance abuse and mental disorders [14]. Tramadol hydrochloride is metabolized in the liver and excreted by the kidney; the role of liver and kidney in drug metabolism predisposes them to toxic injury leading to liver and kidney dysfunction. The result obtained showed that acute and chronic administration could lead to significant increases in the levels of urea, creatinine, MDA, uric acid and activities of AST, ALT and ALP in experimental rabbits. These higher levels observed in tramadol treated rabbits increased with increasing concentrations and duration of administration.
The observed higher levels of uric acid and MDA in rabbits administered with tramadol may be an indication of oxidative stress and lipid peroxidation probably due to generation of excess free radicals more than the available scavenging antioxidant capacity of the body. Due to excessive generation of free radicals, the body synthesizes uric acid in an attempt to mop-up the generated free radicals. Empirical evidence has shown that the production of oxidative radicals in biological systems primarily hydroxyl radicals, peroxy radicals and superoxide anion can damage bio-membranes like lipids, amino acids and nucleic acids. Also, free radicals could denature proteins in kidneys and liver leading to aggregation, loss of function, cross-linking, and destruction of connective tissues [15] as well as leakage of liver enzymes. However, the most destructive effect of oxidative radicals is the induction of lipid peroxidation through the breakdown of poly-unsaturated fatty acid component of cells [16]. Abdel-Zaher et al.[17] reported altered levels of oxidative stress biomarkers in rats administered with tramadol[17]. Several authors have demonstrated oxidative stress induced by tramadol in the brain [8,18-20]. It was reported that electron transfer chain in the mitochondria may be inhibited by tramadol at high doses. In our study, the levels of uric acid and MDA were significantly higher in rabbits administered with 10mg/kg body weight, with levels higher in rabbits administered with 15mg/kg body weight and 20mg/kg body weight. The MDA level and other measured parameters after 15 days of tramadol withdrawal were significantly higher than control group. This is partly consistent with previous study [8]. The authors reported that withdrawal of therapeutic dose of tramadol in male rats resulted in an insignificant decrease in MDA levels. On the contrary, a significant decrease in the levels of MDA was observed after withdrawal of overdose of tramadol in the experimental animals [8]. Elwy and Tab [15] also reported that MDA levels returned back to normal value after 30days of tramadol treatment withdrawal. May be 15 days after withdrawal was insufficient period for the MDA and other parameters to return to levels observed in the control group.
Higher levels of urea and creatinine than control were also observed in this study which is consistent with previous studies [10,20,21,22]. Renal insufficiency due to the decreased glomerular filtration rate characterized by increase oxidative radical production was suggested as possible cause of tramadol-induced nephrotoxicity [23]. Atici et al [10] also reported an increase in biomarkers of renal function in rats with long term tramadol treatment. Alteration in kidney morphology of rats treated with tramadol has been described. The kidney of rats treated with tramadol showed dilated tubules and necrosis of tubular epithelium, after withdrawal of tramadol for 14days, the kidney showed normal histomorphology [11,23].
The acute and chronic administration of tramadol also resulted to significantly higher activity levels of AST, ALT and ALP in experimental rabbits. The hepatic function in drug metabolism involves converting drugs and other compounds into products that are more easily excreted. Metabolites may have a higher activity and/ or a greater toxicity than the original drug. These metabolites, excreted via kidneys, may also cause a cellular damage and/or kidney dysfunction [8]. The liver and kidney are the organs involved in the metabolism and excretion tramadol , whether acute or chronic administration may cause hepatotoxicity and nephrotoxicity [9,10]. The elevated ALT and AST activities observed in response to tramadol administration could be a common sign of impaired liver function [24]. Similarly a significant increase in the levels of ALP was also observed in treated rabbits compared to controls. Impaired liberation of hepatic ALP of liver cell origin may be accompanied by acute cell necrosis, so secretion of ALP in the circulation is increased [8]. The results of the present study were in agreement with that of Senay et al.[25]. Atici, et al.[10] reported that the values of ALT, AST, LDH and Blood urea nitrogen (BUN), and creatinine were significantly increased in opiate group compared to the controls. It was postulated that elevation of hepatic (ALT, AST, LDH) indices could be a secondary event following tramadol-induced lipid peroxidation of hepatocyte with the subsequent increase in the leakage of these biomarkers from the liver [26]. Lipid peroxidation of cell membranes may lead to loss of membrane fluidity, changes in membrane potential and an increase in membrane permeability, all of which lead to leakage of the enzymes from the liver cells. The increased levels of AST, ALP and ALT might indicate tramadol-induced hepatotoxicity. A significant increase in the level of ALT, ALP and AST was seen among animals that received 10 and 20 mg/kg of tramadol for longer duration. The further increase in ALT, ALP and AST activities among animals that were administered tramadol for 30days may indicate the effect of large dose and the duration of drug administration [21].
The limitation of this study is the inability to measure antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and catalase as well as unavailability of liver and kidney histomorphology studies in the rabbits.
Conclusion: The levels of urea, creatinine, uric acid, MDA and the activities of measured liver enzymes were significantly higher in rabbits administered with different concentrations of tramadol. Upon withdrawal of treatment, the levels of the measured variables were still higher than control. The levels of the measured parameters correlated with concentration of administered tramadol and duration of treatment. Tramadol should be administered with caution (whether acute or chronic) in the management of pain and recreational use should be discouraged.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
We appreciate the contributions of all Staff of the Department of Medical Laboratory Science, Achievers University and the Medical Laboratory Scientists in Federal Medical Centre, Owo for their assistance.
References
[1] Andrea, M, Trescot, Sukdeb, Datta, Marion, and Hansen. Opioid Pharmacology. Pain Physician, Opioid Special Issue,2016; 11: 1533-3159.
[2] Tamba BI, Leon MM, Petreus T. Common trace elements alleviate pain in an experimental mouse model. J Neurosci Res. 2013;91:554–61.
[3] Tjaderborn M, Jonsson AK, Ahlner J, Hagg S: Tramadol dependence: a survey of spontaneously reported cases in Sweden. Pharmacoepidemiol Drug Saf 2009;18:1192–8.
[4] Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med. 2005;26:235–44.
[5] Raffa R. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Therapeutics, 2001, 26(4): 257-64.
[6] Emokpae MA, Adesina OO Association of Total Antioxidant status with severity of Anaemia in Pregnancy in Ogun State, Nigeria. J Med Discovery 2018, 3(1) jmd 17055.
[7] Borzelleca,J.F. ,Egle, J.L., Harris, L.S., Johnson, D.N., Terrill, J.B and Belleville, J.A. Toxicological evaluation of mutagonists. Part I: Assessment of toxicity following 30 days of repeated oral dosing of male and female rats with levo-alpha– acetylmethadol HCL (LAAM); J Applied Toxicol 2014; 14 :435–46.
[8] Hussein SA, Abdel-Aal SAL, Ismail HK. Neurodegeneration and oxidative stress induced by tramadol administration in male rats: the effect of its withdrawal. Benha Veterinary Med J 2017;33(2):149-59.
[9] Wu, WN., McKown, LA., Gauthier, AD., Jones, WJ., Raffa RB. Metabolism of analgesic, tramadol hydrochloride, in rat and dog. Xenobiotica 2001; 31(7), 423-41.
[10] Atici, S., Cinel, I., Cinel, L., Doruk, N., Eskandari, G., Oral, U. Liver and kidney toxicity in chronic use of opioids: An experimental long term treatment model. J Biosci 2005; 30(2): 245–52.
[11] Adikwu E, Nelson EC. Assessments of Kidney function and morphology of Tramadol-Diclofenac treated albino rats. Advancements Life Sci, 2018;5(3):104-12.
[12] Poppers, P. J.Hepatic drug metabolism and anesthesia; Anaesthesistics, 2010; 29 : 55–8.
[13] Lee, S.H., Cho, S.Y., Lee, H.G., Choi, J.I., Yoon, M.H and Kim, W.M. Tramadol induced paradoxical hyperalgesia. Pain Physics 2013;16:41 –50.
[14] Nafea, OE., ElKhishin, IA., Awad, OA., Mohamed, DA. A study of the neurotoxic effects of tramadol and cannabis in adolescent male albino rats. Int J Sci Report 2016; 2(7), 143-54.
[15] Elwy, AM., Tabl, G. Effects of Chronic Usage of Tramadol, Acetaminophen and Tramacet on Some Biochemical and Immunological Changes in Male Rats. J Drug Res Egypt 2014; 35(1): 63-71.
[16] Pantelias, K., Grapsa, E. Drug abuse and kidney. Hippokratia 2011;15, 4–8.
[17] Abdel-Zaher, AO., Abdel-Rahman, MS., ELwasei, FM. Protective effect of Nigella sativa oil against tramadol-induced tolerance and de-pendence in mice: Role of nitric oxide and oxidative stress. Neuro Toxicol, 2011; 32: 725-33.
[18] Lemarie, A., Grimm, S. Mutations in the heme b-binding residue of SDHC inhibit assembly of respiratory chain complex II in mammalian cells. Mitochondrion 2009; 9: 254-60.
[19] Mohamed, TM., Abdel Ghaffar, HM., El Husseiny, RM. Effects of tramadol, clonazepam, and their combination on brain mitochondrial complexes. Toxicol Ind Health, 2013; 31(12): 1325-33.
[20] El-Maddawy ZK, El-Ashmawy IM. Hepato-renal and hematological effects of diclofenac sodium in rats. Global J Pharmacol, 2013; 7(2): 123-32.
[21] El-Gaafarawi II., 2006. Biochemical Toxicity Induced By Tramadol AdministrationIn Male Rats. Egyptian J Hosp Med 2006; 23: 353-62.
[22] Yasmeen T, Qureshi GS, Perveen S. Adverse effects of diclofenac sodium on renal parenchyma of adult albino rats. J Pak Med Assoc, 2007; 57(7): 349-51.
[23] Awadalla EA, Salah-Eldin A-E. Histopathological and molecular studies on tramadol mediated hepato-renal toxicity in rats. IOSR J Pharm Biol Sci, 2015; 10(6): 90-102.
[24] Aldalou, A.R, Abdel-Aziz I and Shahwan O. Impact of giving sildenafil (Viagra)/ Tramadol (Tramal) combination on the blood of domestic rabbits. J. Sci. 2014; 3: 162-9.
[25] Senay E. C, Adams, E. H, Geller A, Jnciardi J. A, Munoz A, Schnoll, S.H et al. Physical dependence on Ultram (tramadol hydrochloride)/: both opioid like and a typical withdrawal symptom occur. Drug Alc. Depend,.2003; 69(3):233- 41.
[26] Nehru B and Anand P. Oxidative damage following chronic aluminum exposure in adult and pup rat brains. J Trace Elem. Med Biol, 2005;19: 203–8.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/