J Med Discov (2018); 3(1):jmd17058; DOI:10.24262/jmd.3.1.17058; Received September 21st, 2017, Revised January 11st, 2018, Accepted January 25th, 2018, Published February 01st, 2018.
Abnormal biomarkers of Liver Function in Human Immunodeficiency Virus type 1 infected Subjects without Hepatitis B or C co-infection and their Association with Disease Severity
Mathias Abiodun Emokpae1,*, Joy Oseluese Akhimien1
1Department of Medical Laboratory Science, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, Benin City, Nigeria.
*Correspondence: Dr MA Emokpae Department of Medical Laboratory Science School of Basic Medical Sciences, University of Benin, Benin City, Nigeria. Email:mathias.emokpae@uniben.edu.
Abstract
Abnormal liver function tests s are common in Human Immunodeficiency Virus type 1 (HIV-1) infected individuals, especially in those with hepatitis viruses co-infection. The treatment of HIV infection with highly active antiretroviral therapy (HAART) has reduced the morbidity and mortality of infected individuals but may cause possible adverse drug reactions and hepatotoxicity. In this study we evaluated enzymes biomarkers of liver function in HIV-1 seropositive subjects who are treatment naïve and those who are on HAART for at least 4 months and correlated the values with clusters of differentiation (CD4). A total of 150 subjects were randomly recruited for the study which included; 50 HIV positive subjects HAART-naive (aged 36.4±1.5 years), 50 HIV positive subjects on HAART (aged 38.4±1.6years) and 50 HIV negative (aged 36.4±1.4years) control subjects. Serum liver function enzymes were analyzed using Selectra ProS Chemistry Analyzer (Puteaux, France) and CD4 cell count was determined using Partec cyflow counter (Görlitz, Germany). Serum ALP (p<0.001), AST (p=0.023), ALT (p<0.0.001) and GGTP (p<0.001) were significantly higher in HIV positive subjects than controls while CD4 cell count (p<0.001) was significantly lower in HIV positive subjects than controls. Serum AST (p=0.044) was lower while CD4 (p=0.006) was higher in those on HAART than HAART naïve while ALP, ALT and GGTP activities were not significantly different. There was no significant correlation between any of the measured enzymes and CD4 in HIV positive HAART naïve and HAART subjects. HIV positive subjects are at risk of liver disease but treatment with modern antiretroviral therapy may not contribute to liver pathology in HIV infection without hepatitis B virus (HBV) or hepatitis C virus (HCV) co-infections.
Keywords: CD4 count, Highly active antiretroviral therapy, Human immunodeficiency virus infection, Liver function
Introduction
Human Immunodeficiency Virus (HIV) infection is a major global public health issue with 1.2million deaths recorded from HIV-related causes in 2014 [1]. Approximately 36.9 million people were living with HIV at the end of 2014 with 2 million new infections reported globally in 2014[1]. Sub-Sahara Africa is the most affected region, accounting for 70% of cases globally. The prevalence of HIV among adults aged 15-49years in Nigeria was estimated to be 3.2% [2]. Abnormal liver function test parameters are common in HIV-1 infected individuals. The pathogenic mechanisms of liver injury remain controversial thereby making diagnosis and management difficult [3].HIV infection may be associated with direct inflammation of hepatocytes resulting in liver damage. Antiretroviral therapy, immune-allergic reaction and cytotoxicity exacerbated by underlying liver disease. Other hepatic viruses’ co-infections, neoplasm, parasitic infection and non-antiretroviral therapeutic drugs may also cause liver damage in HIV infection [4]. Co-infection with hepatitis B virus (HBV), hepatitis C virus (HCV) or both could also make situation worse due to cytotoxicity of the viral hepatitis.
The introduction and use of highly active antiretroviral therapy (HAART) in the management of HIV infection has reduced the morbidity and mortality of infected individuals but has raised the concern of possible adverse drug reactions and hepatotoxicity [4]. Antiretroviral drugs that are commonly administered include nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) [5].
Several studies have been carried out elsewhere that revealed the impact of antiretroviral therapy on liver function [3,4]. Before the introduction of HAART, the most common cause of liver dysfunction was AIDS-related neoplasm [6]. However with the advent of HAART, the spectrum of liver disease among HIV infected patients have shifted to concomitant infection with HBV, HCV, medication-related hepatotoxicity, alcohol abuse and non-alcoholic fatty liver disease [7,8]. Hepatotoxicity is a relatively common adverse drug reaction that usually results in the discontinuation of treatment in HIV positive patients. The non-nucleoside reverse transcriptase inhibitors (NNRTIs) such as Niverapine, most commonly result in either direct drug toxicity or hypersensitivity reactions [9]. Nucleoside reverse transcriptase inhibitors (NRTIs) such as Stavudine and Zidovudine have been associated with mitochondrial toxicity due to their ability to inhibit the enzyme, mitochondrial polymerase γ [10].
Studies have also implicated viral hepatitis as the leading cause of death among HIV infected individuals [11,12]. HBV and HCV infections are common among HIV positive patients because of the shared routes of transmission. In a retrospective study involving 200 HIV patients in Benin City, 25.5% tested positive for HBV and 3% tested positive for HCV [13]. HCV co-infection increases progressively to cirrhosis, decompensated liver disease, hepatocellular carcinoma and death [14,15]. HBV co-infection has also been shown to increase the risk of cirrhosis, end-stage liver disease and death especially in patients with low CD4 count and alcohol abuse [16]. Abnormal liver function enzymes (aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGTP)) are common and are reported to occur in 40-60% of patients on the current HAART regimen even without HBV or HCV co-infection [17-19], and the management of abnormal liver function remains a challenge [20]. Emphasis has been placed on liver disease in HIV infected patients co-infected with HBV or HCV infection with little or no attention to other causes of liver diseases in those without HBV or HCV [21,22]. Studies that have associated liver enzymes with disease severity in HIV-infected individuals without HBV or HCV co-infection, are scarce in our setting. In this study we evaluated some biomarkers of liver function in HIV-1 seropositive subjects who are treatment free and those who are on treatment with HAART for at least 4months and correlated the values with clusters of differentiation (CD4).
Subjects and Methods
Study participants
The study participants were confirmed HIV-1 seropositive subjects recruited from the HIV outpatient clinic of the Central Hospital, Benin City. A total of 150 subjects were randomly recruited for the study which included; 50 HIV positive subjects not on HAART treatment (aged 36.4±1.5 years), 50 HIV positive subjects on HAART treatment (aged 38.4±1.6years) and 50 HIV negative healthy individuals (36.4±1.4years) which were used as controls.
Inclusion and exclusion criteria
Clinical and demographic data were obtained from the participants by the use of structured questionnaires. Individuals who were below 18 years of age or with medical history of diabetes, hypertension and tuberculosis were excluded from the study. Those who drink alcohol or smoked were also excluded from the study. The participants were screened for hepatitis B and C infection and those that tested positive to hepatitis B surface antigen (HBsAg) and HCV were excluded from the study.
Ethical Consideration
All participants gave informed consent before enrolment and only those who agreed to be part of the research and met the inclusion and exclusion criteria were recruited in to the study. The study protocol was reviewed and approved by the Edo State Ministry of Health (code HM.1208/112; dated 12th May 2016).
Specimen Preparation
Five ml of venous blood was collected with 2mL dispensed into an EDTA container and 3mLwas emptied into plain plastic container. The blood specimen in the plain container was allowed to clot at room temperature for 30 minutes. It was spun at 3000rpm for 10minutes and the serum was separated into another plain plastic container. The serum was kept frozen at -20oC until analyzed for biomarkers of liver function. The whole blood Sample in the EDTA container was used to analyze for CD4 cell count.
Laboratory analyses
Immunologic Analysis: Hepatitis B surface antigen (HBsAg) and HCV antibody screening were carried out using the rapid antibody screening kits (Clinotech diagnostics, Canada). The HIV status of control subjects was confirmed using two HIV rapid antibody test kits; Determine® and Unigold® kits (Trinity Biotech, USA).
The CD4 cell count was determined using Partec cyflow counter (Görlitz, Germany).
The liver function enzymes (AST, ALT, ALP and GGTP) were assayed using Selectra ProS Chemistry Analyzer (Puteaux, France).
| Subjects | Males | Females | Mean Age |
| HIV positive subjects on ART | 8(16%) | 42(84%) | 38.4±1.67 |
| HIV positive ART naïve subjects | 15(30%) | 35(70%) | 36.4±1.48 |
| Control subjects | 18(36%) | 32(64%) | 36.4±1.41 |
Table 1. Age and sex distribution of the HIV positive and control subjects
Fig. 1 showing the percentage gender distribution of study participants
Statistical analysis
Data were analyzed using Statistical Package for Social Science (SPSS) version 21. Continuous data were given as mean ± standard error of mean, while categorical data were given as percentages. One-way ANOVA was used to compare data in all groups while student t – test was used to compare values in each group with controls. At 95 % confidence interval, 2-tailed p < 0.05 was considered to be statistically significant.
Results
Gender distribution of the study participants showed that 87 (87%) were women while 23(23%) were men. Among the control subjects, 32(64%) were women while 18(36%) were men. The age and gender distribution of the study population is shown in table 1 and figure 1.
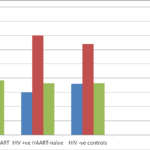
Table 2 and figure 2showed the comparison of the measured variables in the HIV positive subjects (those on HAART and those not on HAART treatments) with the control subjects. Serum ALP (p<0.001), AST (p=0.023), ALT (p<0.0.001) and GGTP (p<0.001) were significantly higher in HIV positive subjects than controls while CD4 cell count (p<0.001) was significantly lower in HIV positive subjects than controls.
| Measured Parameters | HIV positive subjects (n=100) | HIV negative subjects (n=50) | P-values |
| CD4(cells/mm3) | 444.87±33.84 | 939.20±23.67 | 0.000 |
| ALP (U/L) | 230.69±18.12 | 92.76±7.21 | 0.000 |
| AST(U/L) | 27.51±2.62 | 21.08±0.96 | 0.023 |
| ALT(U/L) | 33.50±2.74 | 25.18±1.63 | 0.010 |
| GGT (U/L) | 104.15±18.38 | 35.02±1.39 | 0.000 |
CD4: Cluster of differentiation; ALP: Alkaline phosphatase; ALT: Alanine amino transferase; AST: Aspartate amino transferase; GGT: Gamma glutamyl transpeptidase
Table 2. Comparison of measured variables in the HIV positive subjects with Controls
Fig. 2 showing the comparison of measured parameters between HIV positive and HIV negative subjects.
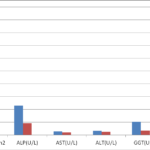
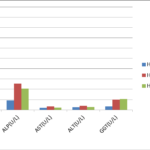
Table 3 and figure 3 showed the comparison of measured liver enzymes activities and CD4 cells between HIV positive subjects on HAART, HAART- naïve and controls. Serum AST (p=0.044) was significantly lower while CD4 (p=0.006) was significantly higher in those on HAART than HAART naïve subjects. Serum ALP, ALT and GGTP activities were not significantly different.
Table 4 showed the correlation of measured enzymes with CD4 count (an index of disease severity) in HIV positive subjects.. There was no significant correlation between any of the measured enzymes with CD4 in HIV positive HAART naïve and HAART subjects.
| Parameters | HIV negative subjects (n=50) | HIV Positive HAART naïve subjects (n=50) | HIV positive subjects on HAART (n=50) | P-Values |
| CD4(cells/mm3) | 939.20±23.67a | 352.10±42.86b | 537.64±49.38be | 0.001 |
| ALP (U/L) | 92.76±7.21a | 254.80±29.13b | 206.58±21.31b | 0.001 |
| AST(U/L) | 21.08±0.96a | 32.76±4.02c | 22.26±3.22cf | 0.023 |
| ALT(U/L) | 25.18±1.63a | 38.32±4.61d | 28.68±2.86ad | 0.010 |
| GGT (U/L) | 35.02±1.39a | 101.32±23.59b | 106.98±28.43b | 0.001 |
CD4: Cluster of differentiation; ALP: Alkaline phosphatase; ALT: Alanine amino transferase; AST: Aspartate amino transferase; GGT: Gamma glutamyl transpeptidase, b=P<0.001; c=P=0.023; d=P=0.010; e=P=0.006;f=0.044;a=P>0.05.
Table 3: Comparison of measured variables in the HIV positive ART naïve, HIV positive on HAART and controls
Fig. 3 showing the comparison of measured parameters among HIV positive HAART naïve, HIV positive on HAART and HIV negative control subjects.
Discussion
The results indicated that liver enzyme activities were significantly higher in HIV positive than HIV negative subjects. On the other hand, ALP, AST and ALT activities were higher in HIV positive HAART naïve than HIV positive on HAART while GGT was higher in HIV positive on HAART than HAART naïve but the different was not statistically significant. There was nonsignificant correlation between CD4 cell counts and measured liver enzymes activities. This may be an indication of no association between abnormal liver enzymes activities and CD4 as well as the use of HAART in HIV management. This observation was consistent with previous studies in sub-Sahara Africa [23,24]. Dusingize et al[23] reported that HIV associated liver damage may occur in HAART-naïve subjects. The authors observed that despite the prevalence of liver transaminase abnormalities were higher in HIV positive subjects with CD4 <200 compared to those with CD4 >350, the difference was not statistically significant [23]. This indicated that elevated liver enzymes are mainly due to HIV infection rather than HIV disease progression as additional contributing factor. Similarly, a study of outpatients in Ugandan observed that the risk of clinically significant hepatotoxicity was low in both HIV positive subjects on HAART and HAART-naïve [24]. The level of enzymes activities in our study is lower than those reported in studies from America, Europe and Asia [3,25-28]. The reason for the differences may be the absence of HBV or HCV co-infection in our study population. It was reported that HIV positive subjects from developed countries are more likely to have chronic hepatitis co-infection which could contribute to severe liver pathologies [29]. Conversely, studies elsewhere suggested that 27% of HIV infected subjects had abnormal liver function tests 6 months after the commencement of HAART [26], which thus indicated that the abnormal LFTs may be due to HAART treatment. Ebot et al[30] in a study of HIV positive subjects in Douala, Cameroon reported that serum ALP and GGT activities in HIV/AIDs patients on HAART were significantly higher than HAART-naïve patients. The authors concluded that both HIV infection and treatment with HAART are associated with liver injury. We did not observe any significant difference in the liver enzymes activities between HIV positive subjects on HAART and HAART naïve subjects. The observed elevation in the activities of measured liver enzymes may be attributed to HIV infection.
| Correlations | R-values | P-Values |
| HIV Positive subjects HAART naive | ||
| CD4/ALP | -0.156 | 0.121 |
| CD4/AST | -0.113 | 0.262 |
| CD4/ALT | -0.268 | 0.07 |
| CD4/GGT | -0.100 | 0.323 |
| HIV Positive subjects on HAART | ||
| CD4/ALP | -0.142 | 0.20 |
| CD4/AST | -0.100 | 0.30 |
| CD4/ALT | -0.248 | 0.09 |
| CD4/GGT | 0.158 | 0.18 |
Table 4: Correlation of CD4 count with the measured variables in HIV positive subjects
Various mechanisms have been proposed to explain liver pathology in HIV infection, such as liver cell apoptosis (induced by Caspase 2,7), mitochondrial dysfunction by decreasing mitochondrial DNA in cells or by changing the mitochondrial membrane by HIV proteins which could cause cytopathic effect on the cells bearing CD4 receptors such as helper T-cells, macrophages, microglial cells, β-lymphocytes, haematopoietic stem cells, rectal mucosal cells and liver sinusoidal epithelial cells [31]. Pathogenesis of liver fibrosis can be explained by direct effect on hepatocytes, hepatic stellate cells and Kupffer cells. Studies have shown that the Kupffer cells of the liver are directly infected by HIV infection both in-vivo [32] and in-vitro[33,34,35] studies. Some authors have suggested that HIV infection of Kupffer cells may result in productive infection [3]. HIV can also influence hepatocyte apoptosis in-vitro via gp120 signaling through CXCR4 in the absence of infection. HIV infection of the gastrointestinal tract may lead to increased permeability of the intestinal walls to bacterial endotoxins such as lipopolysaccharide (LPS). The elevation of LPS has been implicated in liver disease progression in alcoholic liver diseases [36,37]. An elevation in lipopolysaccharides in HIV can stimulate hepatocytes, Kupffer cells and hepatic stellate cells to produce proinflammatory cytokines and chemokines (tumour necrosis factor α, transforming growth factor β and interleukins) which attract activated lymphocytes to the liver and thus drive fibrosis [3]. Kupffer cells are the main cell type in the liver that responds to LPS. Under physiological conditions, Kupffer cells remain tolerant to repeated LPS stimulation but increased LPS contributes to progression of liver disease in HIV-infected patients [38,39].
The nonsignificant correlation between liver enzymes and CD4 cells count among our study population was not in agreement with the study from Tamil Nadu, India [3]. The authors observed significant inverse correlation between CD4 cell count and abnormal LFTs based on their observation of elevated activities of some liver enzymes in HIV positive subjects with different strata of CD4 cell counts.
The limitations of this study are relatively small sample size, majority of the recruited subjects were women because females may be more open than the males in their quest for prevention, treatment and support such that data presented may not reflect the entire population. Also, the baseline data prior to the commencement of ART could not be obtained for HIV positive subjects on HAART due to non-availability of records.
In conclusion, liver enzymes activities in HIV infected subjects were significantly higher in HIV positive subjects than controls, and there was no significant difference in levels of enzymes activities between HIV positive subjects with or without HAART treatment. There was also no significant correlation between liver enzymes activities and CD4 cell counts. HIV positive patients are at risk of liver disease but treatment with modern antiretroviral therapy may not contribute to liver pathology in HIV infection without HBV or HCV co-infections.
Conflict of interest
None
Acknowledgments
We appreciate the contributions of all staff of the Central Hospital, Benin City, Edo State, Nigeria. We are grateful to the Medical Laboratory Scientists for their technical support.
References
1. World Health Organization HIV/AIDS Fact sheet, 2015: http://www.who.int/mediacentre/factsheets/fs360/en/index.html, accessed on 23 April, 2016.
2. UNAIDS. HIV/AIDS estimates, 2014. http://www.unaids.org/en/ regionscountries/countries/nigeria.html, accessed on 26 April, 2016.
3. Savita M, Singh SRB, Vengadakrishnan K, Damodharan J. Liver Function Abnormalities in Human Immunodeficiency Virus Positive Individuals and its Correlation with Disease Severity. Int J Scient Study, 2015; 3(8):15-8.
4. Ramana KV, Ratna R, Sabitha A. Abnormal levels of gamma Glutamyl transpeptidase (GGTP), ALT, AST in Human Immunodeficiency virus-1 (HIV-1) infection. Biochem Physiol. Open Access 2012;1:1-4.
5. Neuman MG, Schnelder M, Nanam RM, Parry C. HIV-Antiretroviral Therapy Induced Liver, Gastrointestinal, and Pancreatic Injury. Int J Hepatol 2012:1-23.
6. Lipsky JJ. Antiretroviral drugs for AIDS. Lancet 1996;348(9030): 800-803.
7. Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol 2008; 48:353–67.
8. Crum-Cianflone N, Collins G, Medina S, Asher D, Campin R, Bavaro M et al.. Prevalence and Factors Associated with Liver Enzyme Abnormalities among HIV-Infected Persons. Clin Gastroenterol Hepatol.2010; 8(2): 183–91.
9. Puoti M, Nasta P, Gatti F, Matti A, Prestini K, Biasi L et al.. HIV Related Liver Disease: ARV Drugs, Coinfection, and other Risk Factors. J Int Assoc Prov AIDS Care 2009; 8(1): 130-42.
10. Price CJ, Thio CL. Liver Disease in the HIV–Infected Individual. Clin Gastroenterol Hepatol 2010; 8:1002-12.
11. Salmon-Ceron D, Lewden C, Morlat P, Be´vilacqua S, Jougla E, Bonnet F et al.. Liver disease as a major cause of death among HIV infected patients:role of hepatitis C and B viruses and alcohol. J Hepatol 2005; 42: 799–805.
12. Weber R, Sabin CA, FriisMøller N, Reiss P, ElSadr WM, Kirk O et al. Liver Related Deaths in Persons Infected With the Human Immunodeficiency Virus: The D:A:D Study. Am Med Assoc J Intern Med 2006;66(15):1632-41.
13. Omonkhelin JO, Egonmwan EA. A review of viral hepatitis in HIV positive patients using UBTH as case Study. Afr J Health Sci 2010;17:1-4.
14. Pineda JA, Macias J. Progression of liver fibrosis in patients coinfected with hepatitis C virus and human immunodeficiency virus undergoing antiretroviral therapy. J Antimicrob Chemotherapy. 2005; 55(4): 417-19.
15. Merchantea N, Giron-Gonzalez JA, Gonzalez-Serranoc M, Torre-Cisnerosd J, Garcıa-Garcıa JA, Arizcorreta A et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS 2006; 20:49–57.
16. Thio CL, Seaberg EC, Skolasky R Jr., Phair J, Visscher B, Munoz A et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360:1921–6.
17. Sterling RK, Chiu S, Snider K, Nixon DO. The prevalence and Risk factors for abnormal enzymes in HIV positive patients without Hepatitis B or C coinfection. Dig Dis Sci 2008;53(5):1-17.
18. Sabin CA. Pitfalls of assessing hepatotoxicity in trials and observational cohorts. Clin Infect Dis.2004; 38 (Suppl 2):S56–S64.
19. Meraviglia P, Schiavini M, Castagna A, Vigano P, Bini T, Landonio S, et al. Lopinavir/ritonavir treatment in HIV antiretroviral-experienced patients: evaluation of risk factors for liver enzyme elevation. HIV Med. 2004; 5(5):334–43.
20. Kottilil S, Polis MA, Kovacs JA. HIV Infection, hepatitis C infection, and HAART: hard clinical choices. JAMA. 2004; 292(2):243–50.
21. Rockstroh JK, Mocroft A, Soriano V, Tural C,Losso MH, Horban A . Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiviral therapy. J Infect Dis 2005; 192:992–1002.
22. Maida I, Nunez M, Rios MJ, Martin-Carbonero L, Sotgiu G, et al. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J AIDS. 2006; 42:177–82.
23. Dusingize JC, Hoover DR, Shi Q, Mutimura E, Rudakemwa E, Ndacyayisenga V et al. Association of Abnormal liver function parameters with HIV serostatus and CD4 count in Antiretroviral-Naïve Rwanda women. AIDS Res Human Retrovir 2015;31(7):723-30.
24. Ocama P, Castelnuovo B, Kamya MR,Kirk GD, Reynolds SJ, Kiragga A et al. Low frequency of liver enzyme elevation in HIV-infected patients attending a large urban treatment centre in Uganda. Int J STD AIDS 2010;21(8):553–7.
25. Clark JM, Brancati FL, and Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98(5):960–7.
26. Crane M, Iser D, and Lewin SR. Human immunodeficiency virus infection and the liver. World J Hepatol 2012;4(3):91–8.
27. Pathania S, Kaur N, Kumar S, Sashindran VK, Puri P. A Cross-sectional study of liver function tests in HIV-infected persons in Western India. Med J Armed Forces Ind 2017;73:23-8.
28. Mata-Marin JA, Gaytan-Martinez J, Grados-Chavarria BH, Fuentes-Allen J, Arroyo-Anduiza CI, Alfaro-Mejia A. Correlation between HIV viral load and aminotransferases as liver damage markers in HIV infected patients: a concordance cross sectional study. Virol J 2009;6:181-4.
29. Prathima MB, Reshma S, Madan GR, Sushith PK, Nair S. Significance of liver enzymes as a baseline investigation in recently diagnosed HIV positive patients. Int J Biomed Adv Res 2015;6(11):768-70.
30. Ebot WO, Achidi EA, Kamga HLF, Njunda AL, Apinjoh TO. Liver function tests of HIV/AIDS patients at the nylon district hospital, Douala, Cameroon. Int J Res Med Sci 2015;3:2549-52.
31. Stebbing J, Gazzard B, Douek DC. Mechanisms of disease – Where does HIV live? N Engl J Med 2004;350: 1872-80.
32. Housset C, Boucher O, Girard PM, Leibowitch J, Saimot AG, Bréchot C, et al. Immunohistochemical evidence for human immunodeficiency virus-1 infection of liver Kupffer cells. Hum Pathol 1990;21:404-8.
33. Hufert FT, Schmitz J, Schreiber M, Schmitz H, Rácz P, von Laer DD. Human Kupffer cells infected with HIV-1 in vivo. J Acquir Immune Defic Syndr 1993;6:772-7.
34. Gendrault JL, Steffan AM, Schmitt MP, Jaeck D, Aubertin AM, Kirn A. Interaction of cultured human Kupffer cells with HIV-infected CEM cells: An electron microscopic study. Pathobiology 1991;59:223-6.
35. Schmitt MP, Gendrault JL, Schweitzer C, Steffan AM, Beyer C, Royer C, et al. Permissivity of primary cultures of human Kupffer cells for HIV-1. AIDS Res Hum Retroviruses 1990;6:987-91.
36. Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW,Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology 2000;32:1008-17.
37. Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2010;7:691-701.
38. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine 2006;12:1365–1371.
39. Schwabe RF, Seki E, Brenner DA. Toll-like receptor signalling in the liver. Gastroenterology 2006;130:1886–1900.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/