J Med Discov (2017);2(3):jmd17032; DOI:10.24262/jmd.2.3.17032; Received July 22nd, 2017, Revised August 30th, 2017, Accepted September 10th, 2017, Published September 12th, 2017.
Adipose Tissue Regulatory T cells Interact with Macrophages – A link between obesity and cancer?
Rui Mao1,*
1Center for Biotechnology and Genomic Medicine, Medical College of Georgia, Augusta University, Augusta, GA 30912
* Correspondence: Rui Mao,Center for Biotechnology and Genomic Medicine,Augusta University,1120, 15th street, Augusta, GA30912, United States;Tel.: +001 706 721 3403, rmao@augusta.edu.
Abstract
Obesity is characterized by abnormal or excessive fat accumulation and chronic low grade inflammation, in adipose tissue. Being one of the worldwide critical health issues, obesity positively correlated with multiple types of cancer and cancer mortality. It is thus utmost important to understand biomolecular and cellular mechanisms underlying the strong positive correlation between obesity and cancer. Adipose tissue is now recognized as an immunological organ where multiple adipose resident immune cells including B cells, T cells, macrophages and adipocytes interact with each other to exert unique immune regulatory functions, and thus, play a key role in the control of inflammation and cancer development. In current review, the role of immune cells in adipose tissue, mainly macrophages and visceral adipose tissue Tregs (VAT Tregs), during the process of obese, inflammation and cancer development was briefly discussed.
Keywords: Obesity,cancer,macrophages,VAT Tregs.
Obesity
Obesity is one of the worldwide critical health issues with abnormal or excessive fat accumulation. Body Mass Index (BMI), defined as the body mass in kilograms divided by the square of the body height in meters (kg/m2), is the most commonly used measure for the classification of overweight and obesity in adults age 20 years or older (Table 1). Based on the report of National Health and Nutrition Examination Survey (NHANES), the vast majority of American adults (70%) are defined as overweight (BMI ≥ 25 kg/m2) and more than 36.5% are classified as obese (BMI ≥ 30 kg/m2) in 2011–2014[1].Overweight and obesity are widely accepted as one of the leading risk factors for global deaths. In a 10 years’ follow‐up cohort study on more than 500 000 US men and women, overweight or obese patients are found to be with increased risk of death by 20-40% to 2-3 folds, respectively, comparing to people of normal weight[2]. This observation is considered mainly due to the fact that overweight and obese subjects are at greater risk for many organic diseases, including diabetes, hypertension, cardiovascular disease and cancer etc, which are responsible for most of the excess deaths[3, 4].
Obesity is associated with increased risk of cancer
It has been reported that overweight and obese positively correlated with multiple cancer types, including endometrial cancer, esophageal adenocarcinoma, multiple myeloma, liver, kidney, pancreatic, colorectal, breast: postmenopausal, gallbladder and ovarian cancer, etc., whereas avoidance of weight gain may prevent subjects from these types of cancer[5, 6]. Based on meta-analyses and/or pooled analyses, the BMI-cancer association was considered to be with a strong dose–response relationship[6]: For example, relative risks of type 1 endometrial cancer are found to be 1.5 for overweight, 2.5 for class I obesity, 4.5 for class II obesity, and 7.1 for class III obesity[7].
In addition, excess bodyweight also contributes to increased cancer mortality: A landmark epidemiological study including more than 900,000 U.S. adults during 16 years of follow-up indicated that increased body weight was associated with increased mortality from a wide range of cancer types which was considered to account for 14 percent of all deaths from cancer in men and 20 percent in women [8].
It is thus utmost important to understand biomolecular and cellular mechanisms underlying the strong positive correlation between obesity and cancer.
| BMI classification | kg/m2 |
| Underweight | <18.5 |
| Normal range | 18.5-24.9 |
| Overweight | ³25 |
| Obese | ³30 |
| Obese Class I | 30-34.9 |
| Obese Class II | 35-40 |
| Obese Class III (Severe obese) | ³40 |
Table 1. BMI classification in adults age 20 years or older.
Biomolecular mechanisms involved in Obesity–Cancer Link
Multiple biological molecules including proinflammatory cytokines, adipokines, insulin and insulin like growth factors, etc. have been proposed to be correlated with obesity-cancer link via certain signaling pathways[9, 10].
Obesity is characterized by a chronic low-grade inflammation with increased level of proinflammatory cytokines including TNFa, IL1, IL6 and IL18, etc. in adipose tissue[11]. There are 2 subtypes of adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT)[12]. Adipose tissue was initially considered only as an inert tissue for lipid storage (stores triacylglicerides, briefly named as TAGs) and energy expenditure, however, WAT was now recognized to be an active endocrine organ which secretes a large amount of bioactive polypeptides known as adipokines. Adipokines (also named as adipocytokines), including adiponectin, leptin, resistin and visfatin, are currently thought to provide an important link between obesity, inflammation, and cancer. Adiponectin exerts anti-inflammatory effect by stimulating some important anti-inflammatory cytokines, such as IL-10 and IL-1 receptor antagonist (IL-1RA) and promotes insulin sensitivity. Adiponectin is found to be decreased whereas leptin is increased in the serum of obese subjects. In consequence, adiponectin has been found to be negatively whereas leptin is positively associated with risk of breast, colon and prostate cancer[13-15].
On the other hand, obesity is tightly associated with type 2 diabetes which is characterized by insulin resistance and hyperinsulinemia. Insulin and IGF-1 pathway has already been linked from obesity, diabetes to cancer[16]. Under physiological circumstances, insulin is produced and secreted by pancreatic islet β cells in response to glucose stimulation and contributes for glucose homeostasis. Insulin can also regulate growth hormone (GH) synthesis, inhibit insulin-like growth factor binding proteins (IGFBPs), and stimulate insulin-like growth factor 1 (IGF-1) production in liver. Increased insulin and IGF1 may inhibit cell apoptosis and promote cancer cell development via PI3K/Akt cell signaling pathway[17, 18]. Enhanced serum IGF-1 level has been reported to be associated with cancer risk by different research groups [19, 20]. On the other hand, exogenous insulin is considered to be a risk factor for cancer development: In type 2 diabetic patient treatment, administration of human insulin is associated with increased cancer risk as compared with insulin analogue treatment (metformin monotherapy)[21].
Cells involved in Obesity–Cancer Link
Myeloid cells (Macrophages, Myeloid-derived suppressor cells, etc.) and lymphoid cells (CD4 and CD8 T cells, Regulatory T cells, NKT cells, B cells, etc.) have been found to be localized in adipose tissue and are named as adipose-resident immune cells. These cells are initially considered to be recruited from circulation and responsible for adipose tissue inflammation and/or infection[22]. The obesity-associated chronic inflammation is characterized by a great infiltration and accumulation of these immune cells from both innate and adaptive immune systems in adipose tissue. Only macrophages and regulatory T cells (Treg) will be discussed in current review.
M1/M2 macrophages
It was believed that tissue-resident macrophages with distinct phenotypes and different functions were key players in maintaining immune homeostasis within adipose tissue. Adipose tissue-macrophages can be subdivided into two major classes: the “classical” macrophages named M1 (pro-inflammatory) and “alternatively activated” M2 (anti-inflammatory), based on their way of activation and different cytokine production profile[23]. M1 macrophages are activated by bacterial moieties (LPS) and/or immune stimuli such as interferon-g (IFNg), produce inflammatory cytokines including TNFa and IL6. In contrast, M2 macrophages are activated by IL4 and produce anti-inflammatory cytokines such as IL10 and TGFb. Under normal conditions, only few macrophages are recruited and adipose tissue presents an anti-inflammatory environment. It was believed that M1 macrophages were recruited and produced pro-inflammatory cytokines at early stage of obese. As obesity progress, more M2 macrophages accumulated in adipose tissue and contributed for chronic inflammation and insulin resistance.
Interestingly, M2 macrophages polarization has been reported to be associated with increased cancer risk, probably by the production of cytokines which can selectively promote regulatory T cell differentiation[23-25].
T cells and Regulatory T cells
Although macrophages are found to be the greatest proportion of leukocytes in adipose tissue and contribute for the chronic inflammation in obese subjects, it has been reported that accumulation of T cells in adipose tissue can precede macrophage infiltration[26-28]. Enhancement of CD4/regulatory T cells (by adoptive transfer) or depletion of CD8 T cells (by specific antibody treatment) in adipose tissue can result in reduction of macrophages infiltration and proinflammatory cytokine production, improvement of insulin sensitivity and glucose homeostasis[26-28]. Therefore, it has been proposed that the infiltration and accumulation of T cells may drive the recruitment of macrophages, determine obesity-associated inflammation in adipose tissue, and finally contribute to insulin resistance and metabolic complications in obese subjects[27, 29, 30].
Moreover, among T lymphocytes in adipose tissue, the balance between regulatory and effector T cell subsets was thought to be key in the control of obesity and disease progression[29]. In both human and mouse studies, much higher level of activated CD8 effector T cells and/or interferon-γ-producing CD4 T helper1 (TH1) cells have been reported in the visceral adipose tissue of obese subjects, as compared to that of CD4 regulatory T (Treg) cells[27, 28].
Regulatory T cell is a special T cell subset that suppress the proliferation and function of multiple immune cell types including CD4 and CD8 T cells, NKT cells, B cells, etc. and thus maintain immune homeostasis in lymphoid organs[31]. A transcriptional factor, Foxp3 (forkhead box P3), can function as a specific marker to represent this cell population since Foxp3 expression has been reported to be tightly associated with development and immune suppressive function of Tregs, by different research groups[32-34]. Recently, the presence of Tregs have been reported in various tissues other than lymphoid organs, such as visceral adipose tissue (VAT Tregs)[26, 35], muscle, intestinal mucosa, placenta, skin, lung and liver, etc. each with unique tissue-specific characteristics [36]. Only VAT Tregs will be discussed in current review.
Visceral adipose tissue Tregs
To date, VAT Tregs are considered as a unique subset with distinct characteristics, as compared to their counterpart conventional Treg cells in lymphoid organs, based on the analysis of phenotype, Treg signature gene expression, T-cell receptor (TCR) repertoire, cytokine production,transcription factors,distinct micro-environment and mechanisms related to their accumulation and activation.
Phenotype
Accumulation of Foxp3+ regulatory T cells has been noticed in visceral adipose tissue of lean, but not obese adult male mice, aged 25-35 weeks old[26]. This specific Treg subset, named as Visceral adipose tissue (VAT) resident Treg cell, accounts for around 50% of CD4 T cell population which is much higher than the fraction (5-15%) normally found in lymphoid (spleen or lymph nodes) or other nonlymphoid (lung, liver) tissues. However, half of infiltrating CD4+T cells may be Treg cells has been reported in some cancer types[37]. Furthermore, VAT Treg cells significantly decreased to normal range in several obese animal model, suggesting that this specific Treg cell population may differ in dynamics and phenotypes in health versus disease condition.
Treg signature gene expression and TCR repertoires
Based on microarray analysis, VAT Tregs reserve approximately 60% of conventional Tregs markers, including CD25, Foxp3, GITR, CTLA-4, OX40 and killer cell lectin-like receptor G1 (KIrg1), etc. which are thought to be directly associated with the development, recognition and function of regulatory T cell population[26].
The origin of VAT Treg cells, has been thought to be either from thymic derived (natural) TR (nTR) cells or peripheral TR (pTR) cells derived from conventional CD4+ T cells with Foxp3 up-regulation. However, VAT Tregs are reported to be characterized by the expression of specific TCR repertoires which are different from those of conventional Tregs, suggesting that most of VAT Tregs may not derive from Tregs in circulation or secondary lymphoid organs[26, 38]. Accumulation of Tregs in adipose tissue may result from in situ proliferation, since an oligo clonal expansion of VAT Tregs was observed by analyzing the TCR repertoire on a single cell level[38].
Cytokines
Activated conventional Tregs can secret a series of cytokines such as IL10 and TGFb, which are thought to be responsible for their immune suppressive function on effector cells[39]. Similarly, VAT Tregs are also characterized by the production of IL10 and TGFb. However, VAT Tregs cells express a much higher level of IL-10 (136-fold augmentation of IL-10 transcripts) and genes downstream of the IL-10 receptor, in comparison with lymph node Treg cells[26]. IL10 can act directly on adipocytes by suppressing TNFα induced inflammatory cytokines and chemokines, and, is considered as one of the key players to prevent obesity associated inflammation and metabolic disorders[26].
Chemokine and adipokine mediation
Distinct profile of chemokines and chemokine receptors has been observed in VAT Tregs: CCR1, CCR2, CCR3, CCR5, CCR9, CXCL1 and CXCL2, CXCR6 are found to be over-expressed while CCR6, CCR7 and CXCR3 are decreased[40]. The specific patterns of chemokine-chemokine receptors expression in VAT Tregs have been proposed to be related to their migration into adipose tissue. However, it was still unclear whether there are difference in the patterns of chemokine and chemokine receptor expression in VAT Tregs between lean and obese subjects?Whether unique type of lean chemokine-chemokine receptor profile contributes to the huge difference in VAT Treg dynamics between lean and obese subjects, in mice?
Besides the biological effect on immune regulation by stimulating pro-inflammatory and anti-inflammatory cytokines, adipokines have been reported to be correlated with Treg proliferation and frequency in adipose tissue: adiponectin might promote whereas leptin probably inhibit Treg proliferation[41].
PPAR-g
VAT Tregs display high level of peroxisome-proliferator-activated receptor g (PPAR-g), a specific transcriptional factor which is thought to be the master regulator of adipocyte differentiation and essential for phenotype, accumulation and function of Treg population in adipose tissue[42]. Based on gene analysis, PPAR-g expression is strongly associated with VAT Treg signature genes[35]. Depletion of PPAR-g in mouse Tregs results in downregulation of almost all VAT Treg signature gene expression and reduction of this cell population specifically in adipose tissue but not lymphoid organs and other non-lymphoid tissues[35]. Furthermore, both PPAR-g isoforms 1 and 2, have been reported to interact with Foxp3 to induce most of the VAT Treg over-expressed genes, and thus contribute for their immune suppressive function[35].
CD36
CD36, a member of the class B scavenger receptor family of cell surface proteins, is expressed on platelets, monocytes, differentiated adipocytes, mammary epithelial cells and macrophages which is responsible for oxidized fatty acid translocation and low-density lipoprotein uptake[42]. VAT Tregs also display high level of CD36 which is thought to be induced by PPAR-g and regarded as a critical mediator for the PPAR-g dependent unique functions of VAT Tregs[43]. Consistently, distinct metabolism has been reported to be closely related to the differentiation of T cell subsets: Th1, Th2, and Th17 cells were highly glycolytic with high expression of glucose transporter Glut1. In contrast, Treg had high level of lipid metabolism with much lower Glut1 level[44].
A hypothesis has been proposed for high frequency of Tregs in adipose tissue in mouse model[40]: In parallel with the process of obesity and macrophage recruitment, VAT Tregs were first recruited into adipose tissue due to chemokine/chemokine receptor recognition and attraction. In response to microenvironment and immune cells in adipose tissue, PPAR-g expression was then increased for displaying unique VAT Treg signature genes and CD36 on the cell surface. The expression of CD36 results in up-regulated fatty acid uptake and lipid metabolism which is in favor of selective differentiation and proliferation of VAT Tregs.
However, contrasting results have been observed in mouse and human studies: As mentioned previously, a significant reduction of VAT Treg cells fraction (15%) was reported in adult obese mice, as compared to lean mice (around 50%), which is correlated with M2 (characterized by IL10 and TGFb production) but not M1 (characterized by IL6 and TNFa production) macrophages[26]. In human study, increased Treg cell population assessed by Foxp3 expression was mainly detected in the adipose tissue of obese subjects, and positively correlated with IL-6 and TNFa expression[45, 46]. Recently, a deep phenotype characterization of regulatory T cells and immune cells was performed by comparing human VAT and blood samples from the same lean and obese subjects, with or without colorectal cancer (CRC)[47]. As compared to peripheral blood (PB), the percentage of Treg/CD4 T cells was not changed (3% vs 5%) whereas the percentage of OX40+/Treg cells was increased (15% vs 3%) in visceral adipose tissue (VAT) stromal vascular fraction (SVF), in normal healthy lean subjects. The percentage of OX40+/Treg cells in SVF was found to be further increased in healthy obese subjects (40% vs 15%), in parallel with slightly increased percentage of total VAT Tregs/CD4 T cells (7.5% vs 3%) [47]. Consistent with previous observation[48], these data suggested that adipose tissue in obese subjects might provide an unique microenvironment (chronic inflammation, etc.) which was selectively in favor of OX40+Treg cell proliferation. OX40 expression is thought to be associated with Treg survival and immune suppressive function, thus, is required for controlling inflammation in adipose tissue characterized by obese subjects[49]. However, it has also been reported that expression and costimulation of OX40+ have detrimental effect on Tregs by reducing Foxp3 expression and thus abrogating Foxp3+ regulatory T cell-mediated suppression[50]. Whether the distinct difference in Treg subset(s) such as OX40+ Tregs within adipose tissue and/or other tissues between healthy lean and obese subjects still remains in other healthy human populations, need to be confirmed and clarified. Different from human, in mice, OX40 is constitutively expressed on all T-regulatory (Treg) cells, including circulating and tissue resident Tregs[51]. Thus, whether the percentage of OX40+ or other specific VAC Treg subsets also increased in healthy obese as compared to lean mice need to be characterized. Furthermore, it is also necessary to figure out which subset(s) of VAT Tregs significantly increased in healthy lean mice and thus results in contradictory finding between human and mouse studies. In addition, percentage of FOXP3/CD4 in circulation was not found to be associated with obese or CRC condition. Surprisingly, increased percentage of VAT OX40+/Treg cells (40%) in healthy obese subjects was found to be significantly decreased in obese CRC patients (20%), which makes a possible correlation between VAT Treg and cancer[47].
Based on these data, a possible mechanism has been proposed for the induction of specific VAT Tregs in human (Figure 1). However, the possible relationship between OX40+ Treg and cancer development still need to be further studied and clarified: OX40 expression on lymphocytes has been found to be negatively correlated with tumor development and several OX40 agonists (OX40 antibody, etc.) are currently tested in cancer clinical trials[48]. Whether VAT Tregs with increased OX40 expression contribute to other obese-associated cancers development, and, via which biological mechanism and cell signaling pathway? Whether the increased expression of OX40 on VAT Tregs results in reduction of Foxp3 and immune suppressive function, leads to block the Treg suppression on antitumor immunity, and finally protect the obese subjects from tumor[50, 51]? Whether the presence of tumor cells affect the phenotype of tissue Tregs by regulating the expression of certain molecules such as OX40+, and finally modulate their immune suppressive function? Or, whether the reduction of VAT Tregs, especially OX40+ VAT Tregs, is simply because of their recruitment at tumor site which provides microenvironment in favor of tumor progression?
Under healthy conditions, similar to conventional Tregs in lymphoid organs, VAT Tregs can suppress the activity of other tissue resident immune cell populations including effector T cells and macrophages, control inflammation and maintain immune homeostasis in adipose tissue. However, obese associated excess VAT Tregs, can greatly inhibit immune reactivity, interact with other adipose resident immune cells and increase susceptibility to pathogen infection such as cancer. Excess VAT Tregs might also be recruited by cancer tissue and contribute for immune escape mechanism and cancer progression.
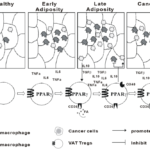
Figure 1. The role of VAT Tregs in the progress of obesity, inflammation and cancer.
In humans, under healthy conditions, only few especially M2 macrophages are recruited and results in an anti-inflammatory environment in adipose tissue. At early stage of obese, M1 macrophages were recruited and produced pro-inflammatory cytokines. As obesity progress, more M1 and M2 macrophages accumulated in adipose tissue and contributed for chronic inflammation. In parallel with the process of obesity and macrophage recruitment, VAT Tregs were first recruited into adipose tissue due to chemokine/chemokine receptor recognition and attraction. In response to microenvironment and immune cells in adipose tissue, PPAR-g expression was increased, leading to increased level of CD36 on the cell surface. The expression of CD36 results in up-regulated fatty acid (FA) uptake and lipid metabolism which is in favor of selective differentiation and proliferation of VAT Tregs. Cytokines secreted by M2 macrophages, such as IL10 and TGFb, also promote the proliferation of VAT Tregs. In humans, increased VAT Tregs are also reported to be positively correlated with IL-6 and TNFα expression. OX40+ Tregs seems to be the specific subset developed in this unique microenvironment which is necessary for suppressing excess inflammation in adipose tissue, at least partially by inhibiting proinflammatory M1 but promoting anti-inflammatory M2 macrophages via high level of IL10 production. Surprisingly, OX40+ VAT Tregs were found to be significantly decreased in obese cancer patients, probably due to migration of VAT Tregs into tumor tissue, and/or, the complicated interaction between immune cells (macrophages and VAT Tregs, etc.) and cancer cells.
Conclusion
Obesity is one of the worldwide critical health issues which is characterized by abnormal or excessive fat accumulation and chronic low-grade inflammation, in adipose tissue. In addition to the classical role in lipid store and energy metabolism, adipose tissue is now recognized as an immunological organ where adipocytes interact with multiple immune cells including B cells, T cells, macrophages to exert unique immune regulatory functions[52]. Increasing evidence from various different research groups suggests an adverse association between obesity and cancer risk/outcomes. The interaction between macrophages and specific Treg population (VAT Tregs) in adipose tissue was briefly discussed in this review, and a simplified possible mechanism was proposed to explain how these cells interact with each other and involved in the process of obese, inflammation and cancer development. Tumor progression is now regarded as a result from the crosstalk between tumor cells and the surrounding “normal” cells, including adipocytes, macrophages, T cells, regulatory T cells, myeloid derived suppressor cells (MDSCs), etc. These surrounding cells are capable of affecting and modifying the cancer cell behavior, vice versa, thus actively involved in tumor progression. Adipose tissue Tregs might represent one of the key immune cell types involved in obesity-cancer link. Future research into how VAT Tregs interact with other tissue resident immune cells, adipocytes and cancer cells may help to provide new strategies to combat obesity-associated chronic inflammation and cancer development.
Conflict of interest
None
Acknowledgments
None
References
- Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD 2016.
- Adams KF, Schatzkin A, Harris TB et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006; 355: 763-78.
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309: 71-82.
- Kitahara CM, Flint AJ, Berrington de Gonzalez A et al. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med 2014; 11: e1001673.
- De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013; 2013: 291546.
- Lauby-Secretan B, Scoccianti C, Loomis D et al. Body Fatness and Cancer–Viewpoint of the IARC Working Group. N Engl J Med 2016; 375: 794-8.
- Setiawan VW, Yang HP, Pike MC et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013; 31: 2607-18.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625-38.
- Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 2012; 1271: 37-43.
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010; 15: 556-65.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111-9.
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 2007; 131: 242-56.
- Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer 2006; 94: 1221-5.
- Ma Y, Yang Y, Wang F et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013; 8: e53916.
- Niu J, Jiang L, Guo W et al. The Association between Leptin Level and Breast Cancer: A Meta-Analysis. PLoS One 2013; 8: e67349.
- Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer 2012; 19: F27-45.
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4: 579-91.
- Gallagher EJ, Fierz Y, Vijayakumar A et al. Inhibiting PI3K reduces mammary tumor growth and induces hyperglycemia in a mouse model of insulin resistance and hyperinsulinemia. Oncogene 2012; 31: 3213-22.
- Chen W, Wang S, Tian T et al. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. Eur J Hum Genet 2009; 17: 1668-75.
- Rinaldi S, Cleveland R, Norat T et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer 2010; 126: 1702-15.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52: 1766-77.
- Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr 2012; 32: 261-86.
- Mantovani A, Sica A, Allavena P et al. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol 2009; 70: 325-30.
- Mantovani A, Sozzani S, Locati M et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549-55.
- Savage ND, de Boer T, Walburg KV et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol 2008; 181: 2220-6.
- Feuerer M, Herrero L, Cipolletta D et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15: 930-9.
- Nishimura S, Manabe I, Nagasaki M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914-20.
- Winer S, Chan Y, Paltser G et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009; 15: 921-9.
- Catalan V, Gomez-Ambrosi J, Rodriguez A, Fruhbeck G. Adipose tissue immunity and cancer. Front Physiol 2013; 4: 275.
- Garg SK, Delaney C, Shi H, Yung R. Changes in adipose tissue macrophages and T cells during aging. Crit Rev Immunol 2014; 34: 1-14.
- Mao R, Xiao W, Liu H et al. Systematic evaluation of 640 FDA drugs for their effect on CD4(+)Foxp3(+) regulatory T cells using a novel cell-based high throughput screening assay. Biochem Pharmacol 2013; 85: 1513-24.
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330-6.
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057-61.
- Workman CJ, Szymczak-Workman AL, Collison LW et al. The development and function of regulatory T cells. Cell Mol Life Sci 2009; 66: 2603-22.
- Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol 2011; 23: 431-7.
- Zhou X, Tang J, Cao H et al. Tissue resident regulatory T cells: novel therapeutic targets for human disease. Cell Mol Immunol 2015; 12: 543-52.
- Samstein RM, Josefowicz SZ, Arvey A et al. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012; 150: 29-38.
- Kolodin DP. Dynamics of Tissue-Resident Regulatory T Cell Populations. Doctoral dissertation, Harvard University 2014.
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8: 523-32.
- Cipolletta D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 2014; 142: 517-25.
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85-97.
- Tontonoz P, Nagy L, Alvarez JG et al. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998; 93: 241-52.
- Sugii S, Olson P, Sears DD et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci U S A 2009; 106: 22504-9.
- Michalek RD, Gerriets VA, Jacobs SR et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 2011; 186: 3299-303.
- Pereira S, Teixeira L, Aguilar E et al. Modulation of adipose tissue inflammation by FOXP3+ Treg cells, IL-10, and TGF-beta in metabolically healthy class III obese individuals. Nutrition 2014; 30: 784-90.
- Zeyda M, Huber J, Prager G, Stulnig TM. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring) 2011; 19: 743-8.
- Donninelli G, Del Corno M, Pierdominici M et al. Distinct Blood and Visceral Adipose Tissue Regulatory T Cell and Innate Lymphocyte Profiles Characterize Obesity and Colorectal Cancer. Front Immunol 2017; 8: 643.
- Weinberg AD, Morris NP, Kovacsovics-Bankowski M et al. Science gone translational: the OX40 agonist story. Immunol Rev 2011; 244: 218-31.
- Piconese S, Timperi E, Pacella I et al. Human OX40 tunes the function of regulatory T cells in tumor and nontumor areas of hepatitis C virus-infected liver tissue. Hepatology 2014; 60: 1494-507.
- Kitamura N, Murata S, Ueki T et al. OX40 costimulation can abrogate Foxp3+ regulatory T cell-mediated suppression of antitumor immunity. Int J Cancer 2009; 125: 630-8.
- Valzasina B, Guiducci C, Dislich H et al. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood 2005; 105: 2845-51.
- Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015; 23: 512-8.
Copyright
© This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/